Abstract
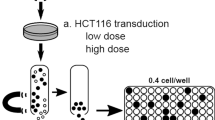
We have developed a highly sensitive polymerase chain reaction (PCR)-based technique termed two-step PCR, which uses arbitrary primers to identify proviral integration sites in retrovirally marked human colony-forming cells. The two-step PCR was established on cell line clones transduced with the SF1m retroviral vector and independently validated by demonstrating identical integration sites with ligation-mediated PCR, a different technique requiring restriction enzyme digestion and adapter ligation for amplifying unknown DNA flanking the provirus. Two-step PCR was performed on peripheral blood progenitor cell (PBPC) colonies that contained as few as 75 cells, which was estimated by quantitative real-time PCR. We were able to amplify and directly sequence proviral integration sites in 35 % of PBPC colonies (25/72, five donors). Identity to the vector long-terminal repeat was confirmed and flanking DNA was found to match with human database sequences, reaffirming specificity. Two-step PCR is a valuable new tool for rapid analysis of genomic target sites for viral vectors, and will aid significantly in understanding clonal development of hematopoiesis and other cell types. Our protocol has the potential for general applicability as the arbitrary primers described here bind to genomic DNA and are thus independent of the vector backbone used.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Abonour R et al. Efficient retrovirus-mediated transfer of the multidrug resistance 1 gene into autologous human long-term repopulating hematopoietic stem cells. Nat Med 2000; 6: 652–658.
Cavazzana-Calvo M et al. Gene therapy of human severe combined immunodeficiency (SCID)-X1 disease1. Science 2000; 288: 669–672.
Li Z et al. Murine leukemia induced by retroviral gene marking. Science 2002; 296: 497.
Guenechea G, Gan OI, Dorrell Cc, Dick JE . Distinct classes of human stem cells that differ in proliferative and self-renewal potential. Nat Immunol 2001; 2: 75–82.
Nolta JA et al. Transduction of pluripotent human hematopoietic stem cells demonstrated by clonal analysis after engraftment in immune-deficient mice. Proc Natl Acad Sci USA 1996; 93: 2414–2419.
Kim HJ et al. Many multipotential gene-marked progenitor or stem cell clones contribute to hematopoiesis in nonhuman primates. Blood 2000; 96: 1–8.
Schmidt M et al. Detection and direct genomic sequencing of multiple rare unknown flanking DNA in highly complex samples. Hum Gene Ther 2001; 12: 743–749.
Laufs S et al. Retroviral vector integration occurs into preferred genomic targets of human bone marrow repopulating cells. Blood 2002; in press.
Lenvik T, Lund TC, Verfaillie CM . Blockerette-ligated capture T7-amplified RT-PCR, a new method for determining flanking sequences. Mol Ther 2002; 6: 113–118.
Sørensen AB, Duch M, Jorgensen P, Pedersen FS . Amplification and sequence analysis of DNA flanking integrated proviruses by a simple two-step polymerase chain reaction method. J Virol 1993; 67: 7118–7124.
Silver J, Keerikatte V . Novel use of polymerase chain reaction to amplify cellular DNA adjacent to an integrated provirus. J Virol 1989; 63: 1924–1928.
Butler SL, Hansen MS, Bushman FD . A quantitative assay for HIV DNA integration in vivo. Nat Med 2001; 7: 631–634.
Sørensen AB et al. Sequence tags of provirus integration sites in DNAs of tumors induced by the murine retrovirus SL3-3. J Virol 1996; 70: 4063–4070.
Rosenthal A, Jones DS . Genomic walking and sequencing by oligo-cassette mediated polymerase chain reaction. Nucleic Acids Res 1990; 18: 3095–3096.
Baum C et al. Novel retrovial vectors for efficient expression of the multidrug resistance (MDR-1) gene in early hematopoietic cells. J Virol 1995; 69: 7541–7547.
Eckert HG et al. High-dose multidrug resistance in primary human hematopoietic progenitor cells transduced with optimized retroviral vectors. Blood 1996; 88: 3407–3415.
Hildinger M et al. FMEV vectors: both retroviral long terminal repeat and leader are important for high expression in transduced hematopoietic cells. Hum Gene Ther 1998; 5: 1575–1579.
Hildinger M et al. Bicistronic retroviral vectors for combining myeloprotection with cell-surface marking. Gene Ther 1999; 6: 1222–1230.
Schiedlmeier B et al. Quantitative assessment of retroviral transfer of the human multidrug resistance 1 gene to human mobilized peripheral blood progenitor cells engrafted in nonobese diabetic/severe combined immunodeficient mice. Blood 2000; 95: 1237–1248.
Baum C et al. Improved vectors for hematopoietic stem cell protection and in vivo selection. J Hematother 1996; 5: 323–329.
Laskus T et al. Detection and sequence analysis of hepatitis B virus integration in peripheral blood mononuclear cells. J Virol 1999; 73: 1235–1238.
Schilz AJ et al. MDR1 gene expression in NOD/SCID repopulating cells after retroviral gene transfer under clinically relevant conditions. Mol Ther 2000; 2: 609–618.
Eckert HG et al. Clinical scale production of an improved retroviral vector expressing the human multidrug resistance 1 gene (MDR1). Bone Marrow Transplant 2000; 25(Suppl 2): 114–117.
Fruehauf S et al. Frequency analysis of multidrug resistance-1 gene transfer into human primitive hematopoietic progenitor cells using the cobblestone area-forming cell assay and detection of vector-mediated P-glycoprotein expression by rhodamine-123. Hum Gene Ther 1996; 7: 1219–1231.
Acknowledgements
The technical assistance of Sigrid Heil, Bernhard Berkus and Hans Jürgen Engel is gratefully acknowledged. We would like to express our gratitude to Prof. Christopher Baum, Hannover, Germany, for providing the retroviral vector used in this investigation. We also thank Dr Klaus Kühlcke, EUFETS GmbH, Idar-Oberstein, Germany, for providing us with retroviral vector stock. The help of Dr Jamal Bullocks, Houston, TX, in reading and correcting the manuscript is highly appreciated. This work was supported in part by grants 10-1294-Ze2 and 10-1710-Ze3 of the Deutsche Krebshilfe/Dr Mildred-Scheel-Stiftung and by grant M 20.4 of the HW & J Hector-Stiftung.
Author information
Authors and Affiliations
Additional information
This article is dedicated to Harald zur Hausen on the occasion of his retirement as head of the German Cancer Research Center (Deutsches Krebsforschungszentrum) in Heidelberg with gratitude and appreciation for 20 years of leadership.
Rights and permissions
About this article
Cite this article
Gentner, B., Laufs, S., Nagy, K. et al. Rapid detection of retroviral vector integration sites in colony-forming human peripheral blood progenitor cells using PCR with arbitrary primers. Gene Ther 10, 789–794 (2003). https://doi.org/10.1038/sj.gt.3301935
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3301935
Keywords
This article is cited by
-
Automated analysis of viral integration sites in gene therapy research using the SeqMap web resource
Gene Therapy (2008)
-
Retroviral vector insertions in T-lymphocytes used for suicide gene therapy occur in gene groups with specific molecular functions
Bone Marrow Transplantation (2006)
-
Retroviral vectors: new applications for an old tool
Gene Therapy (2004)