Abstract
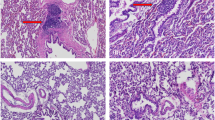
In order to develop a successful gene therapy system for the healing of bone defects, we developed a murine leukemia virus (MLV)-based retroviral system expressing the human bone morphogenetic protein (BMP) 4 transgene with high transduction efficiency. The bone formation potential of BMP4 transduced cells was tested by embedding 2.5 × 106 transduced stromal cells in a gelatin matrix that was then placed in a critical size defect in calvariae of syngenic rats. Gelatin matrix without cells or with untransduced stromal cells were the two control groups. The defect area was completely filled with new bone in experimental rats after 4 weeks, while limited bone formation occurred in either control group. Bone mineral density (BMD) of the defect in the gene therapy group was 67.8 ± 5.7 mg/cm2 (mean ± s.d., n = 4), which was 119 ± 10% of the control BMD of bone surrounding the defect (57.2 ± 1.5 mg/cm2). In contrast, BMD of rats implanted with untransduced stromal cells was five-fold lower (13.8 ± 7.4 mg/cm2, P < 0.001). Time course studies revealed that there was a linear increase in BMD between 2–4 weeks after inoculation of the critical size defect with 2.5 × 106 implanted BMP4 cells. In conclusion, the retroviral-based BMP4 gene therapy system that we have developed has the potential for regeneration of large skeletal defects.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Wozney JM et al. Novel regulators of bone formation: molecular clones and activities Science 1988 242: 1528–1534
Reddi AH . Role of morphogenetic proteins in skeletal tissue engineering and regeneration Nat Biotechnol 1998 16: 247–252
Gerhart TN et al. Healing segmental femoral defects in sheep using recombinant human bone morphogenetic protein Clin Orthop 1993 293: 317–326
Cook SD et al. Effect of recombinant human osteogenic protein-1 on healing of segmental defects in non-human primates J Bone Joint Surg Am 1995 77: 734–750
Zegzula HD et al. Bone formation with use of rhBMP-2 (recombinant human bone morphogenetic protein-2) J Bone Joint Surg Am 1997 79: 1778–1790
Sheehan JP et al. Molecular methods of enhancing lumbar spine fusion Neurosurgery 1996 39: 548–554
Winn SR et al. Gene therapy approaches for modulating bone regeneration Adv Drug Deliv Rev 2000 42: 121–138
Kirker-Head CA . Potential applications and delivery strategies for bone morphogenetic proteins Adv Drug Deliv Rev 2000 43: 65–92
Baltzer AW et al. Genetic enhancement of fracture repair: healing of an experimental segmental defect by adenoviral transfer of the BMP-2 gene Gene Therapy 2000 7: 734–739
Lieberman JR et al. The effect of regional gene therapy with bone morphogenetic protein-2-producing bone-marrow cells on the repair of segmental femoral defects in rats J Bone Joint Surg Am 1999 81: 905–917
Riew KD et al. Induction of bone formation using a recombinant adenoviral vector carrying the human BMP-2 gene in a rabbit spinal fusion model Calcif Tissue Int 1998 63: 357–360
Lou J et al. Effect of bone morphogenetic protein-12 gene transfer on mesenchymal progenitor cells Clin Orthop 1999 369: 333–339
Alden TD et al. In vivo endochondral bone formation using a bone morphogenetic protein 2 adenoviral vector Hum Gene Ther 1999 10: 2245–2253
Musgrave DS et al. Adenovirus-mediated direct gene therapy with bone morphogenetic protein-2 produces bone Bone 1999 24: 541–547
Moutsatsos IK et al. Exogenously regulated stem cell-mediated gene therapy for bone regeneration Mol Ther 2001 3: 449–461
Gonda K et al. Heterotopic ossification of degenerating rat skeletal muscle induced by adenovirus-mediated transfer of bone morphogenetic protein-2 gene J Bone Miner Res 2000 15: 1056–1065
Peng H et al. Development of an MFG-based retroviral vector system for secretion of high levels of functionally active human BMP4 Mol Ther 2001 4: 95–104
Stover ML et al. Bone-directed expression of Col1a1 promoter-driven self-inactivating retroviral vector in bone marrow cells and transgenic mice Mol Ther 2001 3: 543–550
Oreffo RO, Virdi AS, Triffitt JT . Retroviral marking of human bone marrow fibroblasts: in vitro expansion and localization in calvarial sites after subcutaneous transplantation in vivo J Cell Physiol 2001 186: 201–209
Breitbart AS et al. Gene-enhanced tissue engineering: applications for bone healing using cultured periosteal cells transduced retrovirally with the BMP-7 gene Ann Plast Surg 1999 42: 488–495
Schmitz JP et al. Characterization of rat calvarial nonunion defects Acta Anat (Basel) 1990 138: 185–192
Krebsbach PH et al. Gene therapy-directed osteogenesis: BMP-7-transduced human fibroblasts form bone in vivo Hum Gene Ther 2000 11: 1201–1210
Kenley R et al. Osseous regeneration in the rat calvarium using novel delivery systems for recombinant human bone morphogenetic protein-2 (rhBMP-2) J Biomed Mater Res 1994 28: 1139–1147
Lieberman JR et al. Regional gene therapy with a BMP-2-producing murine stromal cell line induces heterotopic and orthotopic bone formation in rodents J Orthop Res 1998 16: 330–339
Engstrand T et al. Transient production of bone morphogenetic protein 2 by allogeneic transplanted transduced cells induces bone formation Hum Gene Ther 2000 11: 205–211
Hollinger JO, Winn SR . Tissue engineering of bone in the craniofacial complex Ann NY Acad Sci 1999 875: 379–385
Sweeney TM et al. Repair of critical size rat calvarial defects using extracellular matrix protein gels J Neurosurg 1995 83: 710–715
Krebsbach PH et al. Repair of craniotomy defects using bone marrow stromal cells Transplantation 1998 66: 1272–1278
Wang J, Glimcher MJ . Characterization of matrix-induced osteogenesis in rat calvarial bone defects: II. Origins of bone-forming cells Calcif Tissue Int 1999 65: 486–493
Hammonds RG Jr et al. Bone-inducing activity of mature BMP-2b produced from a hybrid BMP-2a/2b precursor Mol Endocrinol 1991 5: 149–155
Zhang RW et al. Expression of selected osteogenic markers in the fibroblast-like cells of rat marrow stroma Calcif Tissue Int 1995 56: 283–291
Acknowledgements
This material is based on work supported by a special appropriation to the Jerry L Pettis Memorial VA Medical Center, Musculoskeletal Disease Center. All work was performed with facilities provided by the Department of Veterans Affairs. We thank Dr D Strong for encouragement and helpful discussions and Dr C Nyght for establishing the Western blot procedures. We acknowledge the excellent technical assistance of Yue-hua Liu, Mitra Bhattacharyya, Cheryl Busse and Jann Smallwood.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Gysin, R., Wergedal, J., Sheng, MC. et al. Ex vivo gene therapy with stromal cells transduced with a retroviral vector containing the BMP4 gene completely heals critical size calvarial defect in rats. Gene Ther 9, 991–999 (2002). https://doi.org/10.1038/sj.gt.3301772
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3301772
Keywords
This article is cited by
-
Marrow Stromal Cell-Based Cyclooxygenase 2 Ex Vivo Gene-Transfer Strategy Surprisingly Lacks Bone-Regeneration Effects and Suppresses the Bone-Regeneration Action of Bone Morphogenetic Protein 4 in a Mouse Critical-Sized Calvarial Defect Model
Calcified Tissue International (2009)
-
LMP-1 Retroviral Gene Therapy Influences Osteoblast Differentiation and Fracture Repair: A Preliminary Study
Calcified Tissue International (2008)
-
Gene-enhanced tissue engineering for dental hard tissue regeneration: (1) overview and practical considerations
Head & Face Medicine (2006)
-
Transplantation of skin fibroblasts expressing BMP-2 contributes to the healing of critical-sized bone defects
Journal of Bone and Mineral Metabolism (2006)
-
Bone regeneration in a rat cranial defect with delivery of PEI-condensed plasmid DNA encoding for bone morphogenetic protein-4 (BMP-4)
Gene Therapy (2005)