Abstract
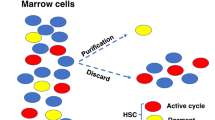
Many exciting discoveries reported over the past 3 years have caused us to expand the paradigm for understanding somatic stem cell plasticity. Within adult organs, there are not only specific stem cells that are capable of producing functional cells of one organ system, but also cells with the flexibility to differentiate into multiple other cell types. In the bone marrow, for example, in addition to hematopoietic stem cells and supportive stromal cells, there are cells with the potential to differentiate into mature cells of the heart, liver, kidney, lungs, GI tract, skin, bone, muscle, cartilage, fat, endothelium and brain. A subpopulation of cells in the brain can differentiate into all of the major cell types in the brain and also into hematopoietic and skeletal muscle cells. In this brief overview, several of these recent findings are summarized.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Krause DS et al. Characterization of murine CD34, a marker for hematopoietic progenitor and stem cells Blood 1994 84: 691–701
Wolf N, Kone A, Priestley G, Bartelmez S . In vivo and in vitro characterization of long-term repopulating primitive hematopoietic cells isolated by sequential Hoechst 33342-rhodamine 123 FACS selection Exp Hematol 1993 21: 614–619
Goodell MA et al. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo J Exp Med 1996 183: 1797–1806
Fleming WH et al. Functional heterogeneity is associated with the cell cycle status of murine hematopoietic stem cells J Cell Biol 1993 122: 897–902
Pereira R et al. Cultured adherent cells from marrow can serve as long-lasting precursor cells for bone, cartilage, and lung in irradiated mice Proc Natl Acad Sci USA 1995 92: 4857–4861
Prockop D . Marrow stromal cells as stem cells for nonhematopoietic tissues Science 1997 276: 71–74
Pereira RF et al. Marrow stromal cells as a source of progenitor cells for nonhematopoietic tissues in transgenic mice with a phenotype of osteogenesis imperfecta Proc Natl Acad Sci USA 1998 95: 1142–1147
Pittenger MF et al. Multilineage potential of adult human mesenchymal stem cells Science 1999 284: 143–147
Friedenstein AJ, Chailakhjan RK, Lalykina KS . The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells Cell Tissue Kinet 1970 3: 393–403
Horwitz EM et al. Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta Nat Med 1999 5: 309–313
Reyes M et al. Purification and ex vivo expansion of postnatal human marrow mesodermal progenitor cells Blood 2001 98: 2615–2625
Ferrari G et al. Muscle regeneration by bone marrow-derived myogenic progenitors Science 1998 279: 1528–1530
Gussoni E et al. Dystrophin expression in the mdx mouse restored by stem cell transplantation Nature 1999 401: 390–394
Kocher AA et al. Neovascularization of ischemic myocardium by human bone-marrow-derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function Nat Med 2001 7: 430–436
Orlic D et al. Bone marrow cells regenerate infarcted myocardium Nature 2001 410: 701–705
Orlic D et al. Mobilized bone marrow cells repair the infarcted heart, improving function and survival Proc Natl Acad Sci USA 2001 98: 10344–10349
Eglitis MA, Mezey E . Hematopoietic cells differentiate into both microglia and macroglia in the brains of adult mice Proc Natl Acad Sci USA 1997 94: 4080–4085
Brazelton TR, Rossi FM, Keshet GI, Blau HM . From marrow to brain: expression of neuronal phenotypes in adult mice Science 2000 290: 1775–1779
Mezey E et al. Turning blood into brain: cells bearing neuronal antigens generated in vivo from bone marrow Science 2000 290: 1779–1782
Sanchez-Ramos J et al. Adult bone marrow stromal cells differentiate into neural cells in vitro Exp Neurol 2000 164: 247–256
Woodbury D, Schwarz EJ, Prockop DJ, Black IB . Adult rat and human bone marrow stromal cells differentiate into neurons J Neurosci Res 2000 61: 364–370
Petersen BE et al. Bone marrow as a potential source of hepatic oval cells Science 1999 284: 1168–1170
Theise ND et al. Derivation of hepatocytes from bone marrow cells in mice after radiation-induced myeloablation Hepatology 2000 31: 235–240
Theise ND et al. Liver from bone marrow in humans Hepatology 2000 32: 11–16
Lagasse E et al. Purified hematopoietic stem cells can differentiate into hepatocytes in vivo Nat Med 2000 6: 1229–1234
Poulsom R et al. Bone marrow contributes to renal parenchymal turnover and regeneration J Pathol 2001 195: 229–235
Imasawa T et al. The potential of bone marrow-derived cells to differentiate to glomerular mesangial cells J Am Soc Nephrol 2001 12: 1401–1409
Krause DS et al. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell Cell 2001 105: 369–377
Alison MR et al. Hepatocytes from non-hepatic adult stem cells Nature 2000 406: 257
Jackson KA et al. Regeneration of ischemic cardiac muscle and vascular endothelium by adult stem cells J Clin Invest 2001 107: 1395–1402
Bjornson CR et al. Turning brain into blood: a hematopoietic fate adopted by adult neural stem cells in vivo Science 1999 283: 534–537
Rietze RL et al. Purification of a pluripotent neural stem cell from the adult mouse brain Nature 2001 412: 736–739
Zuk PA et al. Multilineage cells from human adipose tissue: implications for cell- based therapies Tissue Eng 2001 7: 211–228
Toma JG et al. Isolation of multipotent adult stem cells from the dermis of mammalian skin Nat Cell Biol 2001 3: 778–784
Wilmut I et al. Viable offspring derived from fetal and adult mammalian cells Nature 1997 385: 810–813
Cibelli JB et al. Cloned transgenic calves produced from nonquiescent fetal fibroblasts Science 1998 280: 1256–1258
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Krause, D. Plasticity of marrow-derived stem cells. Gene Ther 9, 754–758 (2002). https://doi.org/10.1038/sj.gt.3301760
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3301760
Keywords
This article is cited by
-
Very small embryonic-like stem cells (VSELs) regenerate whereas mesenchymal stromal cells (MSCs) rejuvenate diseased reproductive tissues
Stem Cell Reviews and Reports (2022)
-
Combining cell therapy with human autologous Schwann cell and bone marrow-derived mesenchymal stem cell in patients with subacute complete spinal cord injury: safety considerations and possible outcomes
Stem Cell Research & Therapy (2021)
-
Long bone mesenchymal stem cells (Lb-MSCs): clinically reliable cells for osteo-diseases
Cell and Tissue Banking (2017)
-
Study on chemotaxis and chemokinesis of bone marrow-derived mesenchymal stem cells in hydrogel-based 3D microfluidic devices
Biomaterials Research (2016)
-
Pilot study: bone marrow stem cells as a treatment for dogs with chronic spinal cord injury
Regenerative Medicine Research (2014)