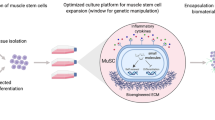
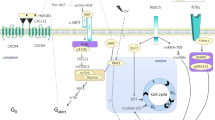
Abstract
The existence of cells with stem cell-like abilities derived from various tissues can now be extended to include the skeletal muscle compartment. Although researchers have focused on the utilization of these cells with regard to their myogenic capacity, initially exploring more efficient cellular therapy treatments for muscular dystrophy, it is becoming increasingly apparent that such cells may one day be used in the treatment of non-myogenic disorders. Evidence regarding the existence and differentiation capacity of muscle-derived stem cells is discussed, along with current theories regarding their proposed position within the myogenic hierarchy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Ham R, Veomett M. . Mechanisms of Development. CV Mosby: St Louis 1980, pp 5–107
Osawa M, Hanada K, Hamada H, Nakauchi H . Long-term lymphohematopoietic reconstitution by a single CD34-low/negative hematopoietic stem cell Science 1996 273: 242–245
Rasko J et al. The flt3/flk-2 ligand: receptor distribution and action on murine haemopoietic cell survival and proliferation Leukemia 1995 9: 2058–2066
Okada S et al. In vivo and in vitro stem cell function of c-kit- and Sca-1-positive murine hematopoietic cells Blood 1992 12: 3044–3050
Mauro A . Satellite cells of skeletal muscle fibers J Biochem Biophys Cytol 1961 9: 493–498
Lipton BH, Schultz E . Developmental fate of skeletal muscle satellite cells Science 1979 205: 1292–1294
Cossu G et al. In vitro differentiation of satellite cells isolated from normal and dystrophic mammalian muscles. A comparison with embryonic myogenic cells Cell Differ 1980 9: 357–368
Bischoff R . The satellite cell and muscle regeneration Engel AG, Franszini-Armstrong C (eds); Myogenesis McGraw-Hill 1994 pp 97–118
Yablonka-Reuveni Z, Rivera AJ . Temporal expression of regulatory and structural muscle proteins during myogenesis of satellite cells on isolated adult rat fibers Dev Biol 1994 164: 588–603
Cornelison D, Wold B . Single-cell analysis of regulatory gene expression in quiescent and activated mouse skeletal muscle satellite cells Dev Biol 1997 191: 270–283
Beauchamp J et al. Expression of CD34 and Myf5 defines the majority of quiescent adult skeletal muscle satellite cells J Cell Biol 2000 151: 1221–1233
Yoshida N et al. Cell heterogeneity upon myogenic differentiation: down-regulation of MyoD and Myf5 generates ‘reserve cells’ J Cell Sci 1998 111: 769–779
Miller J, Schafer L, Dominov J . Seeking muscle stem cells Curr Topic Dev Biol 1999 43: 191–214
Seale P, Rudnicki M . A new look at the origin, function, and ‘stem-cell’ status of muscle satellite cells Dev Biol 2000 218: 115–124
Pate DW et al. Isolation and differentiation of mesenchymal stem cells from rabbit muscle Clin Res 1993 41: 374A
Young HE et al. Pluripotent mesenchymal stem cells reside within avian connective tissue matrices In Vitro Cell Dev Biol Anim 1993 29A: 723–736
Rogers JJ et al. Differentiation factors induce expression of muscle, fat, cartilage, and bone in a clone of mouse pluripotent mesenchymal stem cells Am Surg 1995 61: 231–236
Williams JT et al. Cells isolated from adult human skeletal muscle capable of differentiating into multiple mesodermal phenotypes Am Surg 1999 65: 22–26
Young HE et al. Human pluripotent and progenitor cells display cell surface cluster differentiation markers CD10, CD13, CD56 and MHC class-I Proc Soc Exp Biol Med 1999 221: 63–71
Pittenger MF et al. Multilineage potential of adult human mesenchymal stem cells Science 1999 284: 143–147
Katagiri T et al. Bone morphogenic protein-2 converts differentiation pathway of C2C12 myoblasts into the osteoblast lineage J Cell Biol 1994 127: 1755–1766
Lee J et al. Clonal isolation of muscle-derived cells capable of enhancing muscle regeneration and bone healing J Cell Biol 2000 150: 1085–1099
Bosch P et al. Osteoprogenitor cells within skeletal muscle J Orth Res 2000 18: 933–944
Musgrave DS et al. Ex vivo gene therapy to produce bone using different cell types Clin Orth 2000 378: 290–305
Musgrave DS et al. Ex vivo gene therapy to produce bone using different cell types Adachi N et al. Muscle-derived cell-based ex vivo gene therapy for the treatment of full-thickness articular cartilage defects. J Rheumatol (in press)
Gussoni E et al. Dystrophin expression in the mdx mouse restored by stem cell transplantation Nature 1999 401: 390–394
Jackson KA, Mi T, Goodell MA . Hematopoietic potential of stem cells isolated from murine skeletal muscle Proc Natl Acad Sci USA 1999 96: 14482–14486
Seale P et al. Pax7 is required for the specification of myogenic satellite cells Cell 2000 102: 777–786
Partridge TA, Beauchamp JR, Morgan JE . Conversion of mdx myofibers from dystrophin-negative to positive by injection of normal myoblasts Nature 1989 337: 176–179
Beauchamp JR, Morgan JE, Pagel CN, Partridge TA . Quantitative studies of efficacy of myoblast transplantation Muscle Nerve 1994 18: (Suppl) 261
Huard J et al. Gene transfer into skeletal muscles by isogenic myoblasts Hum Gene Ther 1994 5: 949–958
Fan Y, Maley M, Beilharz M, Grounds M . Rapid death of injected myoblasts in myoblast transfer therapy Muscle Nerve 1996 19: 853–860
Beauchamp JR, Morgan JE, Pagel CN, Partridge TA . Dynamics of myoblast transplantation reveal a discrete minority of precursors with stem cell-like properties as the myogenic source J Cell Biol 1999 144: 1113–1122
Guerette B et al. Control of inflammatory damage by anti-LFA-1: increased success of myoblast transplantation Cell Transplant 1997 6: 101–107
Qu Z et al. Development of approaches to improve cell survival in myoblast transfer therapy J Cell Biol 1998 142: 1257–1267
Cossu G, Mavilio F . Myogenic stem cells for the therapy of primary myopathies: wishful thinking or therapeutic perspective? J Clin Invest 2000 105: 1669–1674
Smythe GM, Hodgetts S, Grounds M . Problems and solutions in myoblast transfer therapy J Cell Mol Med 2001 5: 33–47
Hodgetts S, Beilharz M, Scalzo T, Grounds M . Why do cultured transplanted myoblasts die in vivo? DNA quantification shows enhanced survival of donor myoblasts in host mice depleted of CD4+/CD8+ or NK1.1+ cells Cell Transplant 2000 9: 489–502
Torrente Y et al. Intraarterial injection of muscle-derived CD34+Sca-1+ stem cells restores dystrophin in mdx mice J Cell Biol 2001 152: 335–348
Jankowski R, Haluszczak C, Trucco M, Huard J . Flow cytometric characterization of myogenic cell populations obtained via the preplate technique: potential for rapid isolation of muscle-derived stem cells Hum Gene Ther 2001 12: 619–628
DeAngelis L et al. Skeletal myogenic progenitors originating from embryonic dorsal aorta co-express endothelial and myogenic markers and contribute to post-natal muscle growth and regeneration J Cell Biol 1999 147: 869–878
DeAngelis L et al. Skeletal myogenic progenitors originating from embryonic dorsal aorta co-express endothelial and myogenic markers and contribute to post-natal muscle growth and regeneration Qu-Peterson Z et al. Identification of a novel population of muscle stem cells in mice: potential for muscle regeneration. J Cell Biol (in press)
Acknowledgements
This work was supported in part by grants to Dr Huard from the National Institutes of Health (1P60 AR44811-01, 1PO1 AR45925-01), the Pittsburgh Tissue Engineering Initiative (PTEI), the William F and Jean W Donaldson Chair at Children's Hospital of Pittsburgh, the Muscular Dystrophy Association (USA), the Parent Project (USA), and the Orris C Hirtzel and Beatrice Dewey Hirtzel Memorial Foundation.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Jankowski, R., Deasy, B. & Huard, J. Muscle-derived stem cells. Gene Ther 9, 642–647 (2002). https://doi.org/10.1038/sj.gt.3301719
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3301719
Keywords
This article is cited by
-
Simple and effective serum-free medium for sustained expansion of bovine satellite cells for cell cultured meat
Communications Biology (2022)
-
The latest is not always the greatest
Knee Surgery, Sports Traumatology, Arthroscopy (2022)
-
The role of pulmonary mesenchymal cells in airway epithelium regeneration during injury repair
Stem Cell Research & Therapy (2019)
-
Stem cells on regenerative and reproductive science in domestic animals
Veterinary Research Communications (2019)
-
Scaffold-free tissue engineering for injured joint surface restoration
Journal of Experimental Orthopaedics (2018)