Abstract
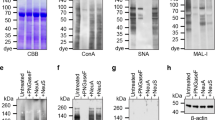
The specificity of lectin binding to distinct saccharides makes them valuable reagents for investigation and identification of cells within complex tissues and potentially for delivery of agents into cells. Therefore we examined lectin binding to airway epithelia. We used an in vitro model of primary cultures of well-differentiated human airway epithelia and applied the lectins to the apical surface of living epithelia. This approach limited binding specifically to the extracellular surface of the apical membrane. Of 32 lectins studied, we found 15 that bound to the apical membrane. The pattern varied from diffuse binding to the surface of nearly all the cells, to binding to a small subset of the cells. Our data combined with earlier studies identify lectins that may be used to detect specific populations of epithelial cells. Because lectins may be used to deliver a variety of agents, including gene transfer vectors, to airway cells, we examined endocytosis of lectins. We found that several lectins bound to the apical surface were actively taken up into the cells. These data may be of value for studies of airway epithelial structure and may facilitate the targeting of the epithelial apical surface.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Schulte BA, Spicer SS . Light microscopic histochemical detection of sugar residues in secretory glycoproteins of rodent and human tracheal glands with lectin-horseradish peroxidase conjugates and the galactose oxidase-Schiff sequence J Histochem Cytochem. 1983 31: 391–403
Spicer SS, Schulte BA, Thomopoulos GN . Histochemical properties of the respiratory tract epithelium in different species Am Rev Respir Dis 1983 128: S20–S26
Saito K, Nakanuma Y . Lectin binding of intrahepatic bile ducts and peribiliary glands in normal livers and hepatolithiasis Tohoku J Exp Med 1990 160: 81–92
Kawai T, Greenberg SD, Titus JL . Lectin histochemistry of normal lung and pulmonary adenocarcinoma Mod Pathol 1988 1: 485–492
Alvarez-Fernandez E, Carretero-Albinana L . Lectin histochemistry of normal bronchopulmonary tissues and common forms of bronchogenic carcinoma Arch Pathol Lab Med 1990 114: 475–481
Sarker AB et al. Lectin histochemistry of normal lung and pulmonary carcinoma Indian J Pathol Microbiol 1994 37: 29–38
Grupp C et al. Identification of nucleated cells in urine using lectin staining Am J Kidney Dis 2001 37: 84–93
El Sherbini H et al. Lectin ligands on human dendritic cells and identification of a peanut agglutinin positive subset in blood Cell Immunol 2000 200: 36–44
Dorscheid DR et al. Characterization of cell surface lectin-binding patterns of human airway epithelium Histochem J 1999 31: 145–151
Honda T et al. Mucosubstance histochemistry of the normal mucosa and epithelial neoplasms of the lung Acta Pathol Jpn 1986 36: 665–680
Mazzuca M, Lhermitte M, Lafitte JJ, Roussel P . Use of lectins for detection of glycoconjugates in the glandular cells of the human bronchial mucosa J Histochem Cytochem 1982 30: 956–966
Byrne P . Lectin binding and tissue fixation Am J Clin Pathol 1987 87: 294–966
Zabner J, Zeiher BG, Friedman E, Welsh MJ . Adenovirus-mediated gene transfer to ciliated airway epithelia requires prolonged incubation time J Virol 1996 70: 6994–7003
Batra RK et al. Receptor-mediated gene delivery employing lectin-binding specificity Gene Therapy 1994 1: 255–260
Yanagihara K, Cheng PW . Lectin enhancement of the lipofection efficiency in human lung carcinoma cells Biochem Biophys Acta 1999 1472: 25–33
Yin W, Cheng PW . Lectin conjugate-directed gene transfer to airway epithelial cells Biochem Biophys Res Commun 1994 205: 826–833
Maples CJ, Ruiz WG, Apodaca G . Both microtubules and actin filaments are required for efficient postendocytotic traffic of the polymeric immunoglobulin receptor in polarized Madin-Darby canine kidney cells J Biol Chem 1997 272: 6741–6751
Gekle M et al. Albumin endocytosis in OK cells: dependence on actin and microtubules and regulation by protein kinases Am J Physiol 1997 272: F668–F677
Welsh MJ . Gene transfer for cystic fibrosis J Clin Invest 1999 104: 1165–1166
Kondo M, Finkbeiner WE, Widdicombe JH . Simple technique for culture of highly differentiated cells from dog tracheal epithelium Am J Physiol 1991 261: L106–L117
Yamaya M, Finkbeiner WE, Chun SY, Widdicombe JH . Differentiated structure and function of cultures from human tracheal epithelium Am J Physiol 1992 262: L713–L724
Yamaya M, Finkbeiner WE, Chun SY, Widdicombe JH . Differentiated structure and function of cultures from human tracheal epithelium
Acknowledgements
We thank Robert Walters, Paola Drapkin, Michael Seiler, Janice Launspach, Tom Moninger, Phil Karp, Pary Weber, Tamara Nesselhauf and Theresa Mayhew for excellent assistance. We thank the In Vitro Cell Models Core for human airway epithelia (supported by the National Heart, Lung, and Blood Institute, the Cystic Fibrosis Foundation, and the National Institutes of Diabetes and Digestive and Kidney Diseases (DK54759)). We especially appreciate the help of ISOPO and IIAM for the human lungs. This work was supported by the National Heart, Lung, and Blood Institute (HL51670). SPY is supported by the Research Training Program in Otolaryngology (US PHS-NIH grant DC00040). MJW is an investigator of the HHMI.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Yi, S., Harson, R., Zabner, J. et al. Lectin binding and endocytosis at the apical surface of human airway epithelia. Gene Ther 8, 1826–1832 (2001). https://doi.org/10.1038/sj.gt.3301598
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3301598
Keywords
This article is cited by
-
Biomimetic Delivery Strategies at the Urothelium: Targeted Cytoinvasion in Bladder Cancer Cells via Lectin Bioconjugates
Pharmaceutical Research (2014)