Abstract
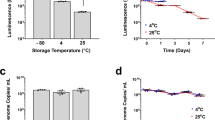
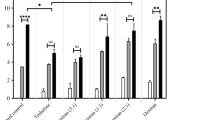
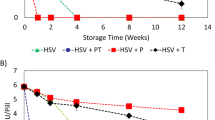
This study summarizes our initial efforts to address an issue that is critical to the success of any multicenter gene therapy clinical trial – maintenance of vector viability during shipping and storage at remote test sites. We have identified formulation and processing factors that influence stability of viral preparations such as selection of appropriate buffer systems, cryoprotectants, and storage conditions. Adenovirus and adeno-associated virus expressing E. coli beta-galactosidase (lacZ) were suspended in blends of complex carbohydrates, cyclodextrins and various surfactants. X-gal stains of 293 and 84-31 cells were used to determine infectious titer of all preparations. Potassium phosphate-buffered preparations consistently maintained high viral titers after storage at −20 and 4°C. Blends of sucrose, mannitol, and surfactant showed negligible loss of titer for 35 days at 4°C. Formulations of sucrose and cyclodextrin were stable for 2 years at −20°C. Negligible loss in titer was observed in unit-dose viral preparations lyophilized in sucrose and stored at 4°C for 1 year after an initial loss of 0.5 log due to processing. Studies with lyophilized sucrose/mannitol blends have shown that viral recovery after processing is directly related to the final moisture content of the dried product. Virus concentration also plays a significant role in recovery after processing with highly concentrated preparations showing minimal loss in titer after lyophilization. In summary, lyophilized preparations that can be shipped and stored at 25°C offer a solution to the current problem of distribution of viral vectors for clinical trials.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Pap T, Gay RE, Gay S . Gene transfer: from concept to therapy Curr Opin Rheumatol 2000 12: 205–210
Nettelbeck DM, Jerome V, Muller R . Gene therapy: designer promoters for tumour targeting Trends Genet 2000 16: 174–181
Hart IR . Tissue specific promoters in targeting systemically delivered gene therapy Semin Oncol 1996 23: 154–158
Clackson T . Regulated gene expression systems Gene Therapy 2000 7: 120–125
Boyd JE . Facilities for large-scale production of vectors under GMP conditions In: Meager A (ed.) Gene Therapy, Technologies, Applications and Regulations John Wiley: Chichester 1999 pp 383–400
O'Riordan CR et al. PEGylation of adenovirus with retention of infectivity and protection from neutralizing antibody in vitro and in vivo Hum Gene Ther 1999 10: 1349–1358
Croyle MA, Chirmule N, Zhang Y, Wilson JM . ‘Stealth’ adenoviruses blunt cell mediated and humoral immune responses against the vector and allow for significant gene expression upon re-administration J Virol 2000 75: 4792–4801
Marshall J et al. Cationic lipid structure and formulation considerations for optimal gene transfection of the lung J Drug Target 2000 7: 453–469
Wang G et al. Increasing epithelial junction permeability enhances gene transfer to airway epithelia in vivo Am J Respir Cell Mol Biol 2000 22: 129–138
Lanuti M et al. Use of protamine to augment adenovirus-mediated cancer gene therapy Gene Therapy 1999 6: 1600–1610
Croyle MA et al. Beta-cyclodextrins enhance adenoviral-mediated gene delivery to the intestine Pharm Res 1998 15: 1348–1355
Wold SM (ed) . Adenovirus Methods and Protocols. Methods in Molecular Medicine Humana Press: Totowa, New Jersey 1999
Croyle MA et al. Factors that influence stability of recombinant adenoviral preparations for human gene therapy Pharm Dev Technol 1998 3: 373–383
Wang W . Instability, stabilization, and formulation of liquid protein pharmaceuticals Int J Pharm 1999 185: 129–188
Murase N, Franks F . Salt precipitation during the freeze-concentration of phosphate buffer solutions Biophys Chem 1989 34: 293–300
Carstensen JT . Drug Stability: Principles and Practices, 2nd edn, Drugs and the Pharmaceutical Sciences vol. 68 Marcel Dekker: New York 1995
International Conference on Harmonization (ICH) Harmonized Tripartite Guideline, Stability Testing of New Drug Substances and Products 1993
Center for Drugs and Biologics FDA, Guidelines Guideline for Submitting Documentation for the Stability of Human Drugs and Biologics. Rockville, MD 1987
Hoener BA, Benet LZ . Factors influencing drug absorption and drug availability In: Banker GS, Rhodes CT (eds) Modern Pharmaceutics Marcel Dekker: New York 1996 pp 121–153
Smolinske SC . Handbook of Food, Drug, and Cosmetic Excipients CRC Press: Boca Raton, FL 1992
Borellini F, Ostrove JM . The transfer of technology from the laboratory to the clinic: in process controls and final product testing In: Meager A (ed.) Gene Therapy Technologies, Applications and Regulations John Wiley: Chichester 1999 pp 359–373
Pikal MJ . Freeze-drying of proteins, part II: formulation selection Biopharm 1990 3: 28–31
Pikal MJ, Dellerman KM, Roy ML, Riggin RM . The effects of formulation variables on the stability of freeze–dried human growth hormone Pharm Res 1991 8: 427–437
Yoshioka S et al. Molecular mobility of protein in lyophilized formulations linked to the molecular mobility of polymer excipients, as determined by high resolution 13C solid-state NMR Pharm Res 1999 16: 35–40
Mattern M, Winter G, Kohnert U, Lee G . Formulation of proteins in vacuum-dried glasses. II. Process and storage stability in sugar-free amino acid systems Pharm Dev Technol 1999 4: 199–208
Sun WQ, Davidson P, Chan HS . Protein stability in the amorphous carbohydrate matrix: relevance to anhydrobiosis Biochim Biophys Acta 1998 1425: 245–254
Carpenter JF, Crowe JH . The mechanism of cryoprotection of proteins by solutes Cryobiology 1988 25: 244–255
Crowe JH et al. Are freezing and dehydration similar stress vectors? A comparison of modes of interaction of stabilizing solutes with biomolecules Cryobiology 1990 27: 219–231
Carpenter JF, Crowe JH . An infrared spectroscopic study of the interactions of carbohydrates with dried proteins Biochemistry 1989 28: 3916–3922
Timasheff SN . The control of protein stability and association by weak interactions with water: how do solvents affect these processes? Ann Rev Biophys Biomol Struct 1993 22: 67–97
Franks F . Freeze-drying: from empericism to predictability Cryo-Letters 1990 11: 93–110
Franks F . Freeze-drying: from empiricism to predictability: the significance of glass transitions Dev Biol Stand 1991 74: 9–19
te Booy MP, de Ruiter RA, de Meere AL . Evaluation of the physical stability of freeze-dried sucrose-containing formulations by differential scanning calorimetry Pharm Res 1992 9: 109–114
Her LM, Nail SL . Measurement of glass transition temperatures of freeze-concentrated solutes by differential scanning calorimetry Pharm Res 1994 11: 54–59
Hatley RH . The effective use of differential scanning calorimetry in the optimization of freeze-drying processes and formulations Dev Biol Stand 1992 74: 105–122
Hatley RH, Franks F . Applications of DSC in the development of improved freeze-drying processes for labile biologicals J Thermal Anal 1991 37: 1905–1914
Pikal MJ . Freeze-drying of proteins. Part 1: process design Biopharm 1990 9: 18–27
Pikal MJ, Dellerman K, Roy ML . Formulation and stability of freeze-dried proteins: effects of moisture and oxygen on the stability of freeze-dried formulations of human growth hormone Dev Biol Stand 1992 74: 21–37
Towns JK . Moisture content in proteins: its effects and measurement J Chromatogr 1995 705: 115–127
Jiang S, Nail SL . Effect of process conditions on recovery of protein activity after freezing and freeze-drying Eur J Pharm Biopharm 1998 45: 249–257
Pristoupil TI, Kramlova M, Fortova H, Ulrych S . Haemoglobin lyophilized with sucrose: the effect of residual moisture on storage Haematologia 1985 18: 45–52
Fitter J . The temperature dependence of internal molecular motions hydrated and dry alpha-amylase: the role of hydration in water in the dynamical transition of proteins Biophys J 1999 76: 1034–1042
Bell LN, Hageman MJ, Bauer JM . Impact of moisture on thermally induced denaturation and decomposition of lyophilized bovine somatotropin Biopolymers 1995 35: 201–209
Greiff D . Stabilities of suspensions of influenza virus dried by sublimation of ice in vacuo to different contents of residual moisture and sealed under different gases Appl Microbiol 1970 20: 935–938
Grieff D . Protein structure and freeze-drying: the effects of residual moisture and gases Cryobiology 1971 8: 145–152
Center for Biologics and Evaluation and Research (CBER). Guidance for Industry Stability Testing of Drug Substances and Drug Products, June 1998. US Department of Health and Human Services, Food and Drug Administration: Rockville, MD.
Graham FL, van der Eb AJ . A new technique for the assay of infectivity of human adenovirus 5 DNA Virology 1973 52: 456–467
Xiao W et al. Gene therapy vectors based on adeno-associated virus type 1 J Virol 1999 73: 3994–4003
Fisher KJ et al. Recombinant adeno-associated virus for muscle-directed gene therapy Nat Med 1997 3: 306–312
Nema S, Avis KE . Freeze-thaw studies of a model protein, lactate dehydrogenase, in the presence of cryoprotectants J Parent Sci Tehcnol 1993 47: 76–83
May JC, Wheeler RM, Etz N, Del Grosso A . Measurement of final container residual moisture in freeze-dried biological products Dev Biol Stand 1992 74: 153–164
May JC, Grim E, Wheeler RM, West J . Determination of residual moisture content in freeze-dried viral vaccines: Karl Fischer, gravimetric, and thermogravimetric methodologies J Biol Stand 1982 10: 249–259
Fisher KJ et al. Transduction with recombinant adeno-associated virus for gene therapy is limited by leading-strand synthesis J Virol 1996 70: 520–532
Croyle MA, Anderson DJ, Roessler BJ, Amidon GL . Development of a highly efficient purification process for recombinant adenoviral vectors for oral gene delivery Pharm Dev Technol 1998 3: 365–372
Acknowledgements
The authors sincerely thank Dr Andrew McGee and Mr William Romanow of the University of Pennsylvania Department of Material Science Engineering and the Laboratory for Research on the Structure of Matter for assistance with differential scanning calorimetry and thermogravimetric analysis. We would also like to thank Animal Models Group (Marcia Houston-Leslie, Rosalind Barr, Jeanna Stabinski, Holly Clouse) of the Institute for Human Gene Therapy for expert technical assistance with vector administration. This work was funded by the Cystic Fibrosis Foundation, the NIH (NHLBI P01 HL59407, NIDDK P30 DK47757), and Genovo, Inc., a biotechnology company which Dr Wilson founded and has equity in. MAC is a recipient of a National Research Service Award.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Croyle, M., Cheng, X. & Wilson, J. Development of formulations that enhance physical stability of viral vectors for gene therapy. Gene Ther 8, 1281–1290 (2001). https://doi.org/10.1038/sj.gt.3301527
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3301527
Keywords
This article is cited by
-
Lyophilization to enable distribution of ChAdOx1 and ChAdOx2 adenovirus-vectored vaccines without refrigeration
npj Vaccines (2023)
-
Stability of enveloped and nonenveloped viruses in hydrolyzed gelatin liquid formulation
Virology Journal (2022)
-
Thermostability and in vivo performance of AAV9 in a film matrix
Communications Medicine (2022)
-
A feasibility study of different commercially available serum-free mediums to enhance lentivirus and adeno-associated virus production in HEK 293 suspension cells
Cytotechnology (2022)
-
Pharmaceutical Development of AAV-Based Gene Therapy Products for the Eye
Pharmaceutical Research (2019)