Abstract
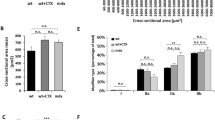
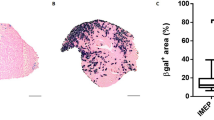
We scrutinized the applicability and efficacy of Sendai virus (SeV) vectors expressing either LacZ or human insulin-like growth factor-I (hIGF-I) in gene transfer into skeletal muscle. Seven days after the intramuscular injection of LacZ/SeV X-gal labeled myofibers were demonstrated in rat anterior tibialis muscle with/without bupivacaine treatment and the transgene expression persisted up to 1 month after injection. Recombinant hIGF-I was detected as a major protein species in culture supernatants of a neonatal rat myoblast cell line L6 and thus induced the cells to undergo myogenetic differentiation. The introduction of hIGF-I/SeV into the muscle showed a significant increase in regenerating and split myofibers which were indicative of hypertrophy, and also an increase in the total number of myofibers, in comparison to that seen in the LacZ/SeV-treated control muscle. These results demonstrate that SeV achieves high-level transgene expression in skeletal muscle, and that hIGF-I gene transfer using SeV vector may therefore have great potential in the treatment of neuromuscular disorders.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Nagai Y . Paramyxovirus replication and pathogenesis. Reverse genetics transforms understanding Rev Med Virol 1999 9: 83–99
Nagai Y, Kato A . Paramyxovirus reverse genetics is coming of age Microbiol Immunol 1999 43: 613–624
Shioda T, Iwasaki K, Shibuta H . Determination of the complete nucleotide sequence of the Sendai virus genome RNA and the predicted amino acid sequence of the F, HN, and L protein Nucleic Acid Res 1986 14: 1545–1563
Kato A et al. Initiation of Sendai virus multiplication from transfected cDNA or RNA with negative of positive sense Genes Cells 1996 1: 569–579
Hasan MK et al. Creation of an infectious recombinant Sendai virus expressing the firefly luciferase gene from the 3′ proximal first locus J Gen Virol 1997 78: 2813–2820
Moriya C et al. Large quantity production with extreme convenience of human SDF-1α and SDF-1β by a Sendai virus vector FEBS Lett 1998 425: 105–111
Florini JR . Hormonal control of muscle growth Muscle Nerve 1987 10: 577–598
Florini JR, Magri KA . Effects of growth factors on myogenic differentiation Am J Physiol 1989 256: C701–C711
Grounds MD . Towards understanding skeletal muscle regeneration Path Res Pract 1991 187: 1–22
Shiotani A et al. Reinnervation of motor endlates and increased muscle fiber size after hIGF-I gene transfer into the paralyzed larynx Hum Gene Ther 1998 9: 2039–2047
Barton-Davis ER et al. Viral mediated expression of insulin growth factor I blocks the aging-related loss of skeletal muscle function Proc Natl Acad Sci USA 1998 95: 15603–15607
Engert JC, Berglund EB, Rosenthal N . Proliferation precedes differentiation in IGF-I-stimulated myogenesis J Cell Biol 1996 135: 431–440
Musaro A, Rosenthal N . Maturation of the myogenic program is induced by postmitotic expression of insulin-like growth factor I Mol Cell Biol 1999 19: 3115–3124
Semsarian C, Sutrave P, Richmond DR, Graham RM . Insulin-like growth factor (IGF-I) induces myotube hypertrophy associated with an increase in anerobic glycolysis in a clonal skeletal-muscle cell model Biochem J 1999 339: 443–451
Delling U et al. A calcineurin-NFATc3-dependent pathway regulates skeletal muscle differentiation and slow myosin heavy-chain expresssion Mol Cell Biol 2000 20: 6600–6611
Sakai Y et al. Accommodation of foreign genes into the Sendai virus genome: sizes of inserted genes and viral replication FEBS Lett 1999 456: 21–226
Jansen M et al. Sequence of cDNA encoding human insulin-like growth factor I precursor Nature 1983 306: 609–611
Alila H et al. Expression of biologically active human insulin-like growth factor-I following intramuscular injection of a formulated plasmid in rats Hum Gene Ther 1997 8: 1785–1795
Rosen KM, Wentworth BM, Rosenthal N, Villa-Komaroff L . Specific temporally regulated expression of the insulin-like growth factor II gene during muscle cell differentiation Endocrinology 1993 133: 474–481
Engert JC, Berglund EB, Rosenthal N . Proliferation precedes differentiation in IGF-I-stimulated myogenesis J Cell Biol 1996 135: 431–440
Florini JR, Ewton DZ . Highly specific inhibition of IGF-I-stimulated differentiation by an antisense oligodeoxyribonucleotide to myogenin mRNA J Biol Chem 1990 265: 13435–13437
Sartore S, Gorza L, Schiaffino S . Fetal myosin heavy chain in regenerating muscle Nature 1982 298: 294–296
Schiaffino S et al. Embryonic and neonatal myosin heavy chain in denervated and paralyzed rat skeletal muscle Dev Biol 1988 127: 1–11
Vitadello M et al. Gene transfer in regenerating muscle Hum Gene Ther 1994 5: 11–18
Merly F et al. Macrophages enhance muscle satellite cell proliferation and delay their differentiation Muscle Nerve 1999 22: 724–732
Lescaudron L et al. Blood borne macrophages are essential for the triggering of muscle regeneration following muscle transplant Neuromusc Disord 1999 9: 72–80
Gonyea W, Ericson GC, Bonde-Petersen F . Skeletal muscle fiber splitting induced by weight-lifting exercise in cats Acta Physiol Scand 1977 99: 105–109
Gonyea W . Muscle fiber splitting in trained and untrained animals Exercise Sports Sci Rev 1980 8: 19–39
Acknowledgements
We acknowledge B. Moss for supplying vTF7-3, and D. Kolakofsky for supplying pGME-N, pGEM-P and pGEM-L.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Shiotani, A., Fukumura, M., Maeda, M. et al. Skeletal muscle regeneration after insulin-like growth factor I gene transfer by recombinant Sendai virus vector. Gene Ther 8, 1043–1050 (2001). https://doi.org/10.1038/sj.gt.3301486
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3301486
Keywords
This article is cited by
-
Advances in RNA Viral Vector Technology to Reprogram Somatic Cells: The Paramyxovirus Wave
Molecular Diagnosis & Therapy (2022)
-
Sendai virus-mediated gene transfer of the c-myc suppressor far-upstream element-binding protein-interacting repressor suppresses head and neck cancer
Gene Therapy (2015)
-
Effect of combined extract of Hansogdan (Phlomis umbrosa) and Dalgaebi (Commelina communis) as a milk additive for enhancing the growth of physical height in vivo
Food Science and Biotechnology (2012)
-
Efficient and stable Sendai virus-mediated gene transfer into primate embryonic stem cells with pluripotency preserved
Gene Therapy (2005)
-
Reduced toxicity of F-deficient Sendai virus vector in the mouse fetus
Gene Therapy (2004)