Abstract
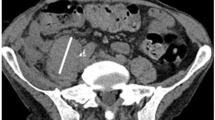
Although genetically engineered adenoviruses hold promise for the treatment of cancer, clinical trial reports have utilized intratumoral injection to date. To determine the feasibility of intravenous delivery of ONYX-015, an E1B-55kD gene-deleted replication selective adenovirus with demonstrated clinical safety and antitumoral activity following intratumoral injection, we performed a clinical trial in patients with metastatic solid tumors. ONYX-015 was infused intravenously at escalating doses of 2 × 1010 to 2 × 1013 particles via weekly infusion within 21-day cycles in 10 patients with advanced carcinoma metastatic to the lung. No dose-limiting toxicity was identified. Mild to moderate fever, rigors and a dose-dependent transient transaminitis were the most common adverse events. Neutralizing antibody titers significantly increased within 3 weeks in all patients. IL-6, γ-IFN, TNF-α and IL-10 increased within 24 h following treatment. Evidence of viral replication was detectable in three of four patients receiving ONYX-015 at doses ⩾2 × 1012 particles and intratumoral replication was confirmed in one patient. In conclusion, intravenous infusion of ONYX-015 was well tolerated at doses up to 2 × 1013 particles and infection of metastatic pulmonary sites with subsequent intratumoral viral replication was seen. The intravenous administration of genetically altered adenovirus is a feasible approach.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Kozarsky K, Grossman M, Wilson JM . Adenovirus-mediated correction of the genetic defect in hepatocytes from patients with familial hypercholesterolemia Somat Cell Mol Genet 1993 19: 449–458
Roth JA, Cristiano RJ . Gene therapy for cancer: what have we done and where are we going? J Natl Cancer Inst 1997 89: 21–39
Martuza RL et al. Experimental therapy of human glioma by means of a genetically engineered virus mutant Science 1991 252: 854–856
Kirn D . Replication-selective micro-organisms: fighting cancer with targeted germ warfare J Clin Invest 2000 105: 836–838
Heise C, Kirn D . Replication-selective adenoviruses for cancer J Clin Invest 2000 105: 847–851
Hawkins L, Nye J, Castro D, Kirn D . Replicating adenoviral gene therapy Proc AACR 1999 40: 476
Freytag SO et al. A novel three-pronged approach to kill cancer cells selectively: concomitant viral, double suicide gene, and radiotherapy Hum Gene Ther 1998 9: 1323–1333
Miller D . Gene therapy on trial Science 2000 288: 951–957
Lechner MS et al. Human papilloma virus E6 proteins bind p53 in vivo and abrogate p53-mediated repression of transcription EMBO J 1992 1: 3045–3052
Gannon JV, Lane DP . p53 and DNA polymerase alpha compete for binding to SV40 T antigen Nature 1987 329: 456–458
Bischoff JR et al. An adenovirus mutant that replicates selectively in p53-deficient human tumor cells Science 1996 274: 373–376
Harris C, Hollstein M . Clinical implications of the p53 tumor-suppressor gene N Engl J Med 1993 329: 1318–1326
Heise C et al. ONXY-015, an E1B gene-attenuated adenovirus, causes tumor-specific cytolysis and antitumoral efficacy that can be augmented by standard chemotherapeutic agents Nature Med 1997 3: 639–645
Goodrum FD, Ornelles DA . The early region 1B 55-kilodalton oncoprotein of adenovirus relieved growth restrictions imposed on viral replication by the cell cycle J Virol 1997 71: 548–561
Turnell AS, Grand RJ, Gallimore PH . The replicative capacities of large E1B-null group A and group C adenoviruses are independent of host cell p53 status J Virol 1999 73: 2074–2083
Rothmann T et al. Replication of ONYX-015, a potential anticancer adenovirus, is independent of p53 status in tumor cells J Virol 1998 72: 9470–9478
Fujiwara T et al. Induction of chemosensitivity in human lung cancer cells in vivo by adenovirus-mediated transfer of the wildtype p53 gene Cancer Res 1994 54: 2287–2291
Heise C et al. Intravenous administration of ONYX-015, a replication-selective adenovirus, induces antitumoral efficacy Cancer Res 1999 59: 2623–2628
You L, Yang C-T, Jablons DM . ONYX-015 works synergistically with chemotherapy in lung cancer cell lines and primary cultures freshly made from lung cancer patients Cancer Res 2000 60: 1009–1013
Khuri F et al. A controlled trial of intratumoral ONYX-015, a selectively replicating adenovirus, in combination with cisplatin and 5-FU in patients with recurrent head and neck cancer Nature Med 2000 6: 879–885
Gordon MS et al. A phase I trial of recombinant human IL-6 in patients with myelodysplastic syndromes and thrombocytopenia Blood 1995 85: 3066–3076
Nemunaitis J et al. Human marrow stromal cells: response to IL-6 and control of IL-6 expression Blood 1989 74: 1929–1935
O'Neil WK . Toxicological comparison of E2A-deleted and first generation adenoviral vectors expressing alpha-1-antitrypsin after systemic delivery Hum Gene Ther 1998 9: 1587–1598
Yang Y, Xiang H, Ertl J, Wilson JM . Upregulation of class I major histocompatibility complex antigens by γ-IFN is necessary for T-cell mediated elimination of recombinant adenovirus-injected hepatocytes in vivo Proc Natl Acad Sci USA 1995 92: 7257–7261
Yang Y, Li Q, Erth HC, Wilson JM . Cellular and humoral immune responses to viral antigens create barriers to lung-directed gene therapy with recombinant adenovirus J Virol 1995 69: 2004–2015
Yang Y, Greenough K, Wilson JM . Transient immune blockade prevents formation of neutralizing antibody to recombinant adenovirus and allows repeated gene transfer to mouse liver Gene Therapy 1996 3: 412–420
Ganly I et al. A phase I study of ONYX-015, an E1B attenuated adenovirus, administered intratumorally to patients with recurrent head and neck cancer Clin Cancer Res 2000 6: 798–806
Worgall S, Wolff G, Falck-Pedersen E, Crystal RG . Innate immune mechanisms dominate elimination of adenoviral vectors following in vivo administration Hum Gene Ther 1997 8: 37–44
Kass-Eisler A et al. Circumventing the immune response to adenovirus-mediated gene therapy Gene Therapy 1996 3: 154–162
Huard J et al. The route of administration is a major determinant of the transduction efficiency of rat tissues by adenoviral recombinants Gene Therapy 1995 2: 107–115
Wolff G et al. Enhancement of in vivo adenovirus-mediated gene transfer and expression by prior depletion of tissue macrophages in the target organ J Virol 1997 71: 624–629
Biewenga J et al. Macrophage depletion in the rat after intra-peritoneal administration of liposome-encapsulated clodronate: depletion kinetics and accelerated repopulation of peritoneal and omental macrophages by administration of Freund's adjuvant Cell Tissue Res 1995 280: 189–196
McCuskey RS, McCuskey PA, Urbaschek R, Urbaschek B . Kupffer cell function in host defense Rev Infect Dis 1999 9: S616–S619
Huitinga I et al. Macrophages in T-cell line-mediated demyelination and chronic relapsing experimental autoimmune encephalomyelitis in Lewis rats Clin Exp Immunol 1995 10: 344–351
Laman JD, Kors N, van Rooijen N, Claassen E . Mechanism of follicular trapping localization of immune complexes and cell remnants after elimination and repopulation of different spleen cell populations Immunology 1990 71: 57–62
Pinto AJ, Stewart D, vanRooijen N, Morahan PS . Selective depletion of liver and splenic macrophages using lyposomes encapsulating the drug dichloromethylene diphosphonate: effects of antimicrobial resistance J Leukoc Biol 1991 49: 579–586
Qian Q, Jutila A, van Rooijen N, Cutler JE . Elimination of mouse splenic macrophages correlates with increased susceptibility to experimental disseminated candidiasis J Immunol 1994 152: 5000–5008
Tscaikowsky D, Brain JD . Effects of liposome-encapsulated dichloromethylene diphosphonate on macrophage function and endotoxin-induced mortality Biochem Biophys Acta 1994 1222: 323–330
van Rooijen N . The liposome-mediated macrophage ‘suicide’ technique J Immunol Meth 1989 124: 1–6
van Rooijen N, Kors N, Kraal G . Macrophage subset repopulation in the spleen differential kinetics after liposome-mediated elimination J Leukoc Biol 1989 45: 97–104
van Rooijen N, Kors N, van der Ende M, Kijkstra CD . Depletion and repopulation of macrophages in spleen and liver of rat after intravenous treatment with liposome-encapsulated dichloromethylene diphosphone Cell Tissue Res 1990 260: 215–222
van Rooijen N, Sanders A . Liposome mediated depletion of macrophages: mechanism of action preparation of lyposomes and application J Immunol Meth 1994 174: 83–93
van Rooijen N, Sanders A . Kupffer cell depletion by liposome-delivered drugs: comparative activity of intracellular clodronate propamidine and ethylenediaminetetraacetic acid Hepatology 1996 23: 1239–1243
Vreden SG et al. Kupffer cell elimination enhances development of liver schizonts of Plasmodium berghei in rats Infect Immunol 1993 61: 1936–1939
Li E, Stupack D, Bokoch GM, Nemerow GR . Adenovirus endocytosis requires actin cytoskeleton reorganization mediated by Rho family GTPases J Virol 1998 72: 8806–8812
Wickham TJ, Mathias P, Cheresh DA, Nemerow GR . Integrins αvβ5 and αvβ5 promote adenovirus internalization but not virus attachment Cell 1993 73: 309–319
Chardonnet Y, Dales S . Early events in the interaction of adenoviruses with HeLa cells. I. Penetration of type 5 and intracellular release of the DNA genome Virology 1970 40: 462–477
Patterson S, Russell WC . Ultrastructural and immunofluorescence studies of early events in adenovirus–HeLa cell interaction J Gen Virol 1983 64: 1091–1099
Li E et al. Adenovirus endocytosis via αv integrins requires phosphoinositide-3-OH-kinase J Virol 1998 72: 2055–2061
Bokoch GM et al. Rac GTPase interacts specifically with phosphatidylinositol 3-kinase Biochem J 1996 315: 775–779
Rodriguez-Viciana P et al. Phosphotidylinositol-3-OH kinase as a direct target of ras Nature 1994 370: 527–532
Tolias KF, Cantley L, Carpenter CL . Rho family GTPases bind to phosphoinositide kinase J Biol Chem 1995 270: 17656–17659
Zhang Y, Bagrodia S, Cerione RA . Activation of phosphoinositide 3-kinase activity by Cdc42Hs binding to p85 J Biol Chem 1994 269: 18727–18730
Li Q et al. Assessment of recombinant adenoviral vectors for hepatic gene therapy Hum Gene Ther 1993 4: 403–409
Kjellen L, Pereira HG . Role of adenovirus antigens in the induction of virus neutralizing antibody J Gen Virol 1968 2: 177–185
Wohlfart C . Neutralization of adenoviruses: kinetics, stoichiometry and mechanisms J Virol 1988 62: 2321–2328
Greber UF, Willetts M, Webster P, Helenius A . Stepwise dismantling of adenovirus 2 during entry into cells Cell 1993 75: 477–486
Bai M, Campisi L, Freimuth P . Vitronectin receptor antibodies inhibit infection of HeLa and A549 cells by adenovirus type 12 but not by adenovirus type 2 J Virol 1994 68: 5925–5932
Goldman MJ, Wilson JM . Expression of αvβ5 integrin is necessary for efficient adenovirus-mediated gene transfer in the human airway J Virol 1995 69: 5951–5958
Wohlfart C, Svensson UK, Everitt E . Interaction between HeLa cells and adenovirus type 2 virions neutralized by different antisera J Virol 1985 56: 896–903
Shenk T . Adenoviridae: the viruses and their replication. In: Fields BN, Knipe DM, Howley PM (eds) Fields Virology Lippincott-Raven: Philadelphia 1996 2111
Yang Y, Ertl HC, Wilson JM . MHC class I-restricted cytotoxic T-lymphocytes to viral antigens destroy hepatocytes in mice-infected with E1-deleted recombinant adenoviruses Immunity 1994 1: 433–442
Barr D et al. Strain related variations in adenovirally mediated transgene expression from mouse hepatocytes in vivo: comparisons between immunocompetent and immunodeficient inbred strains Gene Therapy 1995 2: 151–155
Dai Y et al. Cellular and humoral immune response to adenoviral vectors containing factor IX gene: tolerization of factor IX and vector antigens allows for long-term expression Proc Natl Acad Sci USA 1995 92: 1401–1405
Zsengeller ZK et al. Persistence of replication-deficient adenovirus-mediated gene transfer in lung of immune-deficient (nu/nu) mice Hum Gene Ther 1995 6: 457–467
Kass-Eisler A et al. The impact of development stage, route of administration and the immune system on adenovirus-mediated gene transfer Gene Therapy 1994 1: 395–402
Kay MA et al. Long-term hepatic adenovirus-mediated gene expression in mice following CTLA41g administration Nat Genet 1995 11: 191–197
Crystal RG et al. Administration of an adenovirus containing the human CFTR cDNA to the respiratory tract of individuals with cystic fibrosis Nat Genet 1994 8: 42–51
Yang Y, Trinchieri G, Wilson JM . Recombinant IL-12 prevents formation of blocking IfA antibodies to recombinant adenovirus and allows repeated gene therapy to mouse lung Nature Med 1995 1: 890–893
Kolls JK et al. Use of transient CD4 lymphocyte depletion to prolong transgene expression of E1-deleted adenoviral vectors Hum Gene Ther 1996 7: 489–497
Sawchuk SJ et al. Anti-T-cell receptor monoclonal antibody prolongs transgene expression following adenovirus-mediated in vivo gene transfer to mouse synovium Hum Gene Ther 1996 7: 499–506
Bouvet M et al. Suppression of the immune response to an adenovirus vector and enhancement of intratumoral transgene expression by low dose etoposide Gene Therapy 1998 5: 189–195
Fang B . Gene therapy for hemophilia B: host immunosuppression prolongs the therapeutic effect of adenovirus-mediated factor IX expression Hum Gene Ther 1995 6: 1039–1044
Jooss K, Ertl HC, Wilson JM . Cytotoxic T-lymphocyte target proteins and their major histocompatibility complex class I restriction in response to adenovirus vectors delivered to mouse liver J Virol 1998 72: 2945–2954
Engelhardt JF, Ye X, Doranz B, Wilson JM . Ablation of E2A in recombinant adenoviruses improves transgene persistence and decreases inflammatory response in mouse liver Proc Natl Acad Sci USA 1994 91: 6196–6200
Vilquin JT et al. FK506 immunosuppression to control the immune reactions triggered by first-generation adenovirus-mediated gene transfer Hum Gene Ther 1995 6: 1391–1401
Lochmuller H et al. Immunosuppression by FK506 markedly prolongs expression of adenovirus-delivered transgene in skeletal muscles of adult dystrophic (mdx) mice Biochem Biophys Res Commun 1995 213: 569–574
Wold WS, Hermiston TW, Tollefson AE . Adenovirus proteins that subvent host defenses Trends Microbiol 1994 2: 437–443
Lee MG, Abina MA, Haddada H, Perricaudet M . The constitutive expression of the immunomodulatory gp 19k protein in E1-, E3 adenoviral vectors strongly reduces the host cytotoxic T-cell response against the vector Gene Therapy 1995 2: 256–262
Wold WS, Tollefson AE, Hermiston TW . E3 transcription unit of adenovirus Curr Top Microbiol Immunol 1995 199: 237–274
Bett AJ, Haddara W, Prevec L, Graham FL . An efficient and flexible system for construction of adenovirus vectors with insertions or deletions in early regions 1 and 3 Proc Natl Acad Sci USA 1994 91: 8802–8806
Ilan Y et al. Insertion of the adenoviral E3 region into a recombinant viral vector prevents antiviral humoral and cellular immune responses and permits long-term gene expression Proc Natl Acad Sci USA 1997 94: 2587–2592
Horwitz MS, Tufariello J, Grunhaus A . Model system for studying the effects of adenovirus E3 gene on virulence in vivo Curr Top Microbiol Immunol 1995 199: 195–211
Rawle FC, Tollefson AE, Wold WS, Gooding LR . Mouse anti-adenovirus cytotoxic T-lymphocytes. Inhibition of lysis by E3 gp19K but not E1 14.7K J Immunol 1989 143: 2031–2037
Wold WS, Gooding LR . Region E3 of adenovirus: a cassette of genes involved in host immunosurveillance and virus-cell interactions Virology 1991 184: 1–8
Feuerbach D, Burgert HG . Novel proteins associated with MHC class I antigens in cells expressing the adenovirus protein E3/19K EMBO J 1993 12: 3153–3161
Beier DC et al. Association of human class I MHC alleles with the adenovirus E8/19K protein J Immunol 1994 152: 3862–3872
Kaplan JM et al. Characterization of factors involved in modulating persistence of transgene expression from recombinant adenovirus in the mouse lung Hum Gene Ther 1997 8: 45–56
Medina DJ et al. Adenovirus-mediated cytotoxicity of chronic lymphocytic leukemia cells Blood 1999 94: 3499–3508
Rodriguez R et al. Prostate attenuated replication competent adenovirus (ARCA) CN706: a selective cytotoxic for prostate-specific antigen-positive prostate cancer cells Cancer Res 1997 57: 2559–2563
Barker DD, Berk AJ . Adenovirus proteins from both E1B reading frames are required for transformation of rodent cells by viral injection and DNA transfection Virology 1987 156: 107–121
Acknowledgements
We wish to thank Ana Petrovich for manuscript preparation, Sherry Toney for extensive time and effort in coordinating study samples and results as well as editorial proofing of the manuscript, and George Smith and Carrie LeDuc from Althea for providing extensive assistance in determining ONYX-015 DNA analysis.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Nemunaitis, J., Cunningham, C., Buchanan, A. et al. Intravenous infusion of a replication-selective adenovirus (ONYX-015) in cancer patients: safety, feasibility and biological activity. Gene Ther 8, 746–759 (2001). https://doi.org/10.1038/sj.gt.3301424
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3301424
Keywords
This article is cited by
-
A bibliometric review of oncolytic virus research as a novel approach for cancer therapy
Virology Journal (2021)
-
Ad5/3 is able to avoid neutralization by binding to erythrocytes and lymphocytes
Cancer Gene Therapy (2021)
-
Two roads for oncolytic immunotherapy development
Journal for ImmunoTherapy of Cancer (2019)
-
Phase I trial of intravenous Ad5CRT in patients with liver metastasis of gastrointestinal cancers
Cancer Gene Therapy (2019)
-
Ki67 targeted strategies for cancer therapy
Clinical and Translational Oncology (2018)