Abstract
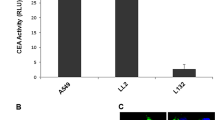
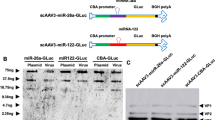
In pancreatic cancer, the mutation of c-K-ras is a critical event of tumor growth and metastasis. We have previously demonstrated a dominant negative effect of N116Y on the growth of pancreatic cancer cells. To evaluate the potential of N116Y for suppressing the metastatic growth of pancreatic tumor cells, we made a replication-deficient recombinant N116Y adenovirus driven by the carcinoembryonic antigen (CEA) promoter (Ad CEA-N116Y). We demonstrated that the expression of N116Y, growth inhibition, and apoptotic death induction were all specific to pancreatic cancer cell lines (PCI-35 and PCI-43) that were promoter positive, whereas no growth retardation was observed in human embryonic pancreas-derived cell line 1C3D3 after Ad CEA-N116Y infection. We examined the effect of Ad CEA-N116Y on the metastatic growth of PCI-43 colonies in liver, which was generated by tumor injection into the spleen of nude mice. The results showed that Ad CEA-N116Y effectively reduced the number of metastatic colonies without any complication by injecting intrasplenically 5 days after tumor cell inoculation. Thus, N116Y can selectively suppress the metastatic growth of pancreatic tumor cell by using the CEA promoter-driven adenovirus vector indicating that N116Y gene therapy may be potentially useful for the treatment of pancreatic cancer patients with liver micrometastasis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Warshaw AL, Fernandez-Del Castillo C . Pancreatic carcinoma New Engl J Med 1992 326: 455–465
Shichinohe T et al. Suppression of pancreatic cancer by the dominant negative ras mutant, N116Y J Surg Res 1996 66: 125–130
Wanebo HJ, Vezeridis MP . Pancreatic carcinoma in perspective Cancer 1996 78: 580–591
Muchmore JH, Preslan JE, George WJ . Regional chemotherapy for inoperable pancreatic carcinoma Cancer Suppl 1996 78: 664–673
Griffin JF et al. Patterns of failure after curative resection of pancreatic carcinoma Cancer 1990 66: 56–61
Tamagawa E et al. Pancreatic lymph nodal and plexus micrometastases detected by enriched polymerase chain reaction and nonradioisotopic single-strand conformation polymorphism analysis: a new predictive factor for recurrent pancreatic carcinoma Clin Cancer Res 1997 3: 2143–2149
Almoguera C et al. Most human carcinomas of the exocrine pancreas contain mutant c-K-ras genes Cell 1988 53: 549–554
Shibata D et al. Detection of c-K-ras mutations in fine needle aspirates from human pancreatic adenocarcinomas Cancer Res 1990 50: 1279–1283
Barbacid M . Ras genes Annu Rev Biochem 1987 56: 779–827
Gansauge S, Gansauge F, Beger HG . Molecular oncology in pancreatic cancer J Mol Med 1996 74: 313–320
Howe JR, Conlon KC . The molecular genetics of pancreatic cancer Surg Oncol 1997 6: 1–18
Lemoine NR . Molecular advances in pancreatic cancer Digestion 1997 58: 550–556
Khwaja A et al. Matrix adhesion and Ras transformation both activate a phosphoinositide 3-OH kinase and protein kinase B/Akt cellular survival pathway EMBO J 1997 16: 2783–2793
Inoue S et al. Detection of hepatic micrometastasis in pancreatic adenocarcinoma patients by two-stage polymerase chain reaction/restriction fragment length polymorphism analysis Jpn J Cancer Res 1995 86: 626–630
Kohl NE et al. Selective inhibition of ras-dependent transformation by a farnesyltransferase inhibitor Science 1993 260: 1934–1937
Aoki K, Yoshida T, Sugimura T, Terada M . Liposome-mediated in vivo gene transfer of antisense K-ras construct inhibits pancreatic tumor dissemination in the murine peritoneal cavity Cancer Res 1995 55: 3810–3816
Kainuma O et al. Inhibition of growth and invasive activity of human pancreatic cancer cells by a farnesyltransferase inhibitor, Manumycin Pancreas 1997 15: 379–383
Clanton DJ, Hattori S, Shih TY . Mutations of the ras gene product p21 that abolish guanine nucleotide binding Proc Natl Acad Sci USA 1986 83: 5076–5080
Ogiso Y et al. Trans-dominant suppressor mutations of the H-ras oncogene Cell Growth Differ 1990 1: 217–224
Ogiso Y et al. Suppression of various human tumor cell lines by a dominant negative H-ras mutant Gene Therapy 1994 1: 403–407
Sakai N et al. Induction of apoptosis by a dominant negative H-ras mutant (N116Y) in K562 cells Exp Cell Res 1994 215: 131–136
Senmaru N et al. Suppression of Erk activation and in vivo growth in esophageal cancer cells by the dominant negative Ras mutant, N116Y Int J Cancer 1998 78: 366–371
Kopper L, Hanh TV, Lapis K . Experimental model for liver metastasis formation using Lewis lung tumor J Cancer Res Clin Oncol 1982 103: 31–38
Kishimoto T et al. Phenotypes correlating to metastatic properties of pancreas adenocarcinoma in vivo: the importance of surface sialyl Lewis antigen Int J Cancer 1996 69: 290–294
Osaki T et al. Gene therapy for carcinoembryonic antigen-producing human lung cancer cells by cell type-specific expression of herpes simplex virus thymidine kinase gene Cancer Res 1994 54: 5258–5261
Schrewe H et al. Cloning of the complete gene for carcinoembryonic antigen: analysis of its promoter indicates a region conveying cell type-specific expression Mol Cell Biol 1990 10: 2738–2748
Tanaka T et al. Adenovirus-mediated prodrug gene therapy for carcinoembryonic antigen-producing human gastric carcinoma cells in vitro Cancer Res 1996 56: 1341–1345
Lan KH et al. Tumor-specific gene expression in carcinoembryonic antigen-producing gastric cancer cells using adenovirus vectors Gastroenterology 1996 111: 1241–1251
Tanaka T et al. Adenovirus-mediated gene therapy of gastric carcinoma using cancer specific gene expression in vivo Biochem Biophys Res Commun 1997 231: 775–779
Lan KH et al. In vivo selective gene expression and therapy mediated by adenoviral vectors for human carcinoembryonic antigen-producing gastric carcinoma Cancer Res 1997 57: 4279–4284
Takeuchi M, Kondo S, Sugiura H, Katoh H . Pre-operative predictors of short-term survival after pancreatic cancer resection Hepatogastroenterology 1998 45: 2399–2403
DiMaio JM et al. Directed enzyme pro-drug gene therapy for pancreatic cancer in vivo Surgery 1994 116: 205–213
Richards CA, Austin EA, Huber BE . Transcriptional regulatory sequences of carcinoembryonic antigen: identification and use with cytosine deaminase for tumor-specific gene therapy Hum Gene Ther 1995 6: 881–893
Yang Y et al. Cellular immunity to viral antigens limits E1-deleted adenoviruses for gene therapy Proc Natl Acad Sci USA 1994 91: 4407–4411
Nicolson GL . Cancer progression and growth: relationship of paracrine and autocrine growth mechanisms to organ preference of metastasis Exp Cell Res 1993 204: 171–180
Yamamoto H et al. Expression of matrix metalloproteinase matrilysin (MMP-7) was induced by activated Ki-ras via AP-1 activation in SW1417 colon cancer cells J Clin Lab Anal 1995 9: 297–301
Anderson SC et al. p53 gene therapy in a rat model of hepatocellular carcinoma: intra-arterial delivery of a recombinant adenovirus Clin Cancer Res 1998 4: 1649–1659
Raper SE et al. Selective gene transfer into the liver of non-human primates with E1-deleted, E2A-defective, or E1-E4 deleted recombinant adenoviruses Hum Gene Ther 1998 9: 671–679
Clayman GL et al. Adenovirus-mediated p53 gene transfer in patients with advanced recurrent head and neck squamous cell carcinoma J Virol 1998 70: 2221–2232
Gao G, Yang Y, Wilson JM . Biology of adenovirus vectors with E1 and E4 deletions for liver-directed gene therapy J Clin Oncol 1996 16: 8934–8943
Engelhardt JF, Ye X, Doranz B, Wilson JM . Ablation of E2A in recombinant adenoviruses improves transgene persistence and decreases inflammatory response in mouse liver Proc Natl Acad Sci USA 1994 91: 6196–6200
Hitt M et al. Techniques for human adenovirus vector construction and characterization. In: Adolph KW (ed)Methods in Molecular Genetics, Vol 7B Academic Press: Florida 1995 pp 13–30
Wong H, Anderson WD, Cheng T, Riabowol KT . Monitoring mRNA expression by polymerase chain reaction: the primer-dropping method Anal Biochem 1994 223: 251–258
Pollman M, Yamada T, Horiuchi M, Gibbons GH . Vasoactive substances regulate vascular smooth muscle cell apoptosis Circ Res 1996 79: 748–756
Matsushita H et al. Inhibition of growth of human vascular smooth muscle cells by overexpression of p21 gene through induction of apoptosis Hypertension 1998 31: 493–498
Couffinhal T et al. Histochemical staining following LacZ gene transfer underestimates transfection efficiency Hum Gene Ther 1997 8: 929–934
Acknowledgements
We thank Dr Karl Riabowol for critical reading of the manuscript. This work was supported in part by a Grants-in-Aid for Scientific Research from the Ministry of Education, Science, Sports and Culture, Japan.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Takeuchi, M., Shichinohe, T., Senmaru, N. et al. The dominant negative H-ras mutant, N116Y, suppresses growth of metastatic human pancreatic cancer cells in the liver of nude mice. Gene Ther 7, 518–526 (2000). https://doi.org/10.1038/sj.gt.3301125
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3301125
Keywords
This article is cited by
-
An Automated High-throughput Array Microscope for Cancer Cell Mechanics
Scientific Reports (2016)
-
Ras-dependent carbon metabolism and transformation in mouse fibroblasts
Oncogene (2006)
-
Transcriptional targeting of adenovirus vectors with the squamous cell carcinoma-specific antigen-2 promoter for selective apoptosis induction in lung cancer
Cancer Gene Therapy (2006)
-
Tissue MicroArray Analyses of Pancreatic Duodenal Homeobox‐1 in Human Cancers
World Journal of Surgery (2005)
-
Trials of gene therapy for pancreatic carcinoma
Current Gastroenterology Reports (2005)