Abstract
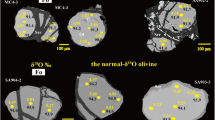
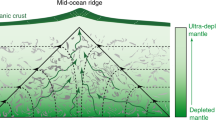
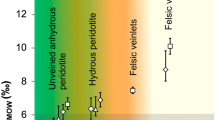
The subduction of oceanic lithosphere is thought to enrich the mantle in elements concentrated in altered oceanic crust and its sedimentary cover (for example, H2O, CO2 and alkalis)1,2. This enrichment is generally inferred from the geochemistry of island-arc lavas3. More direct evidence—such as samples from the mantle with a clear crustal origin4—is rare. Inclusions of silicate glass within mantle-derived minerals can have major- and trace-element compositions unlike basalt5,6, and sometimes contain ‘enriched’ isotopic compositions of Sr, Nd and Pb, suggesting that the inclusions are partial melts of subducted oceanic crust or sediments6. Alternatively, some of these alkali-rich inclusions may have been produced by melting peridotites to low degrees (possibly in the presence of volatiles)7. Here we present oxygen isotope data from silicate glass inclusions obtained from mantle olivine samples in an island-arc setting. These data provide direct evidence that the inclusions are derived from a source rich in material from the subducted oceanic crust and therefore that slab-derived fluids have infiltrated the sub-arc mantle wedge.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Leeman, W. P., Carr, M. J. & Morris, J. D. Boron geochemistry of the Central American volcanic arc: constraints on the genesis of subduction-related magmas. Geochim. Cosmochim. Acta 58, 149–168 (1994).
Stolper, E. M. & Newman, S. The role of water in the petrogenesis of Mariana trough magmas. Earth Planet. Sci. Lett. 121, 293–325 (1994).
Morris, J. & Tera, F. 10Be and 9Be in mineral separates and whole rocks from volcanic arcs: implications for sediment subduction. Geochim. Cosmochim. Acta 53, 3197–3206 (1989).
Daniels, L. R. M., Gurney, J. J. & Harte, B. Acrustal mineral in a mantle diamond. Nature 379, 153–156 (1996).
Schiano, P. & Clocchiatti, R. Worldwide occurrence of silica-rich melts in sub-continental and sub-oceanic mantle minerals. Nature 368, 621–624 (1994).
Hauri, E. H., Schimizu, N., Dieu, J. J. & Hart, S. R. Evidence for hotspot-related carbonatite metasomatism in the oceanic upper mantle. Nature 365, 221–227 (1993).
Baker, M. B., Hirschmann, M. M., Ghiorso, M. S. & Stolper, E. M. Compositions of near-solidus peridotite melts from experiments and thermodynamic calculations. Nature 375, 308–311 (1995).
Ito, E., White, W. M. & Gopel, C. The O, Sr, Nd and Pb isotope geochemistry of MORB. Chem. Geol. 62, 157–176 (1987).
Eiler, J. M. et al. Oxygen isotope variations in ocean island basalt phenocrysts. Geochim. Cosmochim. Acta 61, 2281–2293 (1997).
Mattey, D., Lowry, D. & Macpherson, C. Oxygen isotope composition of mantle peridotite. Earth Planet. Sci. Lett. 128, 231–241 (1994).
Muehlenbachs, K. in Stable Isotopes in High Temperature Geological Processes(eds Valley, J. W., Taylor, H. P. & O'Neill, J. R.) 425–444 (Mineralogical Society of America, Washington DC, 1986).
Arthur, M. A., Anderson, T. F. & Kaplan, I. R. in SEPM Short Course no. 10(SEPM, Tulsa, 1986).
Kolodny, Y. & Epstein, S. Stable isotope geochemistry of deep sea cherts. Geochim. Cosmochim. Acta 40, 1195–1209 (1976).
McInnes, B. I. A. & Cameron, E. M. Carbonated, alkaline hybridizing melts from the sub-arc environment: mantle wedge samples from the Tabar–Lihir–Tanga–Feni arc, Papua New Guinea. Earth Planet. Sci. Lett. 122, 125–141 (1994).
Taylor, B. Bismarck sea: evolution of a back-arc basin. Geology 7, 171–174 (1979).
Taylor, H. P. Jr & Epstein, S. Relationship between 18O/16O ratios in coexisting minerals of igneous and metamorphic rocks. Geol. Soc. Am. Bull. 73, 461–480 (1962).
Palin, J. M., Epstein, S. & Stolper, E. M. Oxygen isotope partitioning between rhyolitic glass/melt and CO2: an experimental study at 550–950 °C and 1 bar. Geochim. Cosmochim. Acta 60, 1963–1973 (1996).
Eiler, J. M., Valley, J. W., Graham, C. M. & Baumgartner, L. P. Ion microprobe evidence for the mechanisms of stable isotope retrogression in high-grade metamorphic rocks. Contrib. Mineral. Petrol. 118, 365–378 (1995).
Spivack, A. J. & Edmond, J. M. Boron isotope exchange between seawater and the oceanic crust. Geochim. Cosmochim. Acta 51, 1033–1043 (1987).
McCulloch, M. T. & Gamble, J. A. Geochemical and geodynamical constraints on subduction zone magmatism. Earth Planet. Sci. Lett. 102, 358–374 (1991).
Green, T. H. Experimental studies of trace-element partitioning applicable to igneous petrogenesis–Sedonia 16 years later. Chem. Geol. 117, 1–36 (1994).
Staudigel, H., Plank, T., White, W. & Schminke, H. U. Geochemical fluxes during seafloor alteration of the basaltic upper oceanic crust: DSDP sites 417 and 418 (overview). AGU Geophys. Monogr. 96, 19–38 (1996).
Salters, V. J. M. & Shimizu, N. World-wide occurrence of HFSE-depleted mantle. Geochim. Cosmochim. Acta 52, 2177–2182 (1988).
Sharma, M. & Wasserburg, G. J. The neodymium isotopic compositions and rare earth patterns in highly depelted ultramafic rocks. Geochim. Cosmochim. Acta 60, 4537–4550 (1996).
Johnson, K. T. M., Dick, H. J. B. & Shimizu, N. Melting in the oceanic upper mantle: an ion microprobe study of diopsides in abyssal peridotites. J. Geophys. Res. 95, 2661–2678 (1990).
Rampone, E. et al. Trace element and isotope geochemistry of depleted peridotites from an N-MORB type ophiolite (Internal Liguride, N. Italy). Contrib. Mineral. Petrol. 123, 61–76 (1996).
Brenan, J. M., Shaw, H. F. & Ryerson, F. J. in 7th Annual Goldschmidt Conference 36 (Tucson, AZ, USA, 1997).
Schneider, M. E. & Eggler, D. H. Fluids in equilbrium with peridotite minerals: implications for mantle metasomatism. Geochim. Cosmochim. Acta 50, 711–724 (1986).
Keppler, H. Cosntraints from partitioning experiments on the composition of subduction-zone fluids. Nature 380, 237–240 (1996).
Ryan, J. G., Morris, J., Tera, F., Leeman, W. P. & Tsvetkov, A. Cross-arc geochemical variations in the Kurile arc as a function of slab depth. Science 270, 625–627 (1995).
Draper, D. S. & Green, T. H. P–T phase relations of silicic, alkaline, aluminous mantle-xenolith glasses under anhydrous and C-O-H fluid-saturated conditions. J. Petrol. 38, 1187–1224.
Brenan, J. M., Shaw, H. F., Ryerson, F. J. & Phinney, D. L. Mineral–aqueous fluid partitioning of trace elements at 900 °C and 2.0GPa: constraints on the trace element chemistry of mantle and deep crustal fluids. Geochim. Cosmochim. Acta 59, 3331–3350 (1995).
Hawkesworth, C. J., Galllagher, K., Hergt, J. M. & McDermott, F. Destructive plate margin magmatism: geochemistry and melt generation. Lithos 33, 169–188 (1994).
Eiler, J. M., Graham, C. M. & Valley, J. W. SIMS analysis of oxygen isotopes: matrix effects in complex minerals and glasses. Chem. Geol. 138, 221–244 (1997).
McDonough, W. F. & Sun, S. S. The composition of the Earth. Chem. Geol. 120, 223–253 (1995).
Plank, T. & Langmuir, C. The chemical composition of subducting sediment and its consequences for the crust and mantle. Chem. Geol.(in the press).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Eiler, J., McInnes, B., Valley, J. et al. Oxygen isotope evidence for slab-derived fluids in the sub-arc mantle. Nature 393, 777–781 (1998). https://doi.org/10.1038/31679
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/31679
This article is cited by
-
Metallogenetic model of Jiaodong-type gold deposits, eastern China
Science China Earth Sciences (2023)
-
Decoupling between Oxygen and Radiogenic Isotopes: Evidence for Generation of Juvenile Continental Crust by Partial Melting of Subducted Oceanic Crust
Journal of Earth Science (2021)
-
Do Supercontinent-Superplume Cycles Control the Growth and Evolution of Continental Crust?
Journal of Earth Science (2020)
-
Serpentinization of New Caledonia peridotites: from depth to (sub-)surface
Contributions to Mineralogy and Petrology (2020)
-
Heavy oxygen recycled into the lithospheric mantle
Scientific Reports (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.