Abstract
Study design:
Retrospective study.
Objectives:
To assess in the long-term clinical and urodynamic results of intraurethral stents in a group of patients with spinal cord injury.
Setting:
Spinal Cord Injury Unit, Juan Canalejo Hospital, A Coruña, Spain.
Methods:
Forty-seven consecutive male patients were studied from 1993 to 2002. All of them suffered from hyperreflexia with detrusor–sphincter dyssynergia (DSD) owing to spinal cord injury, and were treated by means of the placement of an intraurethral stent at the external sphincter.
Results:
After surgery, significant decreases in all the parameters studied were observed. The number of patients with symptoms of urinary tract infection decreased by 25% (P<0.031). Post-void residual urine volume experienced an average decrease of 224.3 cm3 (P=0.001). Episodes of dysreflexia decreased from 35.1 to 16.2% (P=0.039). The urodynamic study showed an average reduction of 44.36 cm H2O in the maximum detrusor pressure (P<0.0001). Complications in the upper urinary tract descended from 46.8 to 23.4% after placing the stent (P=0.013). The most frequent stent complication was displacement, followed by stenosis, lithiasis and intraprosthetic calcification. In all, 8.5% required the stent removal.
Conclusions:
Intraurethral stent is a good choice for the long-term management of DSD in spinal cord-injured patients, even in those who had been previously submitted to prior sphincterotomy. It has the advantage of being a potentially reversible procedure, so patients prefer it to more invasive therapies such as sphincterotomy.
Similar content being viewed by others
Introduction
Detrusor–sphincter dyssynergia (DSD) is a common cause of obstruction of bladder outlet in patients suffering from spinal cord injury (SCI). This leads to an increase in intravesical pressure, as well as incomplete bladder emptying, which is connected to numerous complications: recurrent urinary tract infections (UTI), autonomic dysreflexia, vesicouretheral reflux (VUR), hydronephrosis, etc. Situations which, if left untreated, may lead to high intrarenal pressures resulting in renal failure.1 Renal failure has been the most common cause of death among people with SCI. Presently, it has gone from being the first cause of mortality to the fourth position.2 However, despite the fact that the management of these patients has improved a lot in the last few years, the ideal treatment has still not been found for DSD in male patients.3
External sphincterotomy remains the mainstay of treatment to achieve a reduction in the resistance of the urinary tract in this situation,4 as it has proven its efficiency in the prevention of complications of the upper urinary tract (UUT) caused by DSD.5
In those patients with an adequate manual ability, a programme of intermittent self-catheterization, generally associated to anticholinergic treatment, is the best method for bladder management.6 Most quadriplegic patients lack the skills required to perform catheterisations, whereas some of them who do have these skills refuse to use them. For these patients and for those in whom urinary emptying is not achieved at low pressure, or who do not tolerate oral medication, this surgical technique is recommended.7 Nevertheless, the morbidity of this procedure, even if it has been gradually reduced with the improvements in the technique (location of incisions, laser sphincterotomy, etc), is still a problem. It includes complications like bleeding (0–13%),8, 9, 10 technique failures (12–26%),11 obstruction of the bladder neck or urethral stenosis (3–13%),10, 12, 13 re-intervention (15–40%)9, 12, 14, 15 and erectile dysfunction (3–7% with incisions at 12 O'clock position, higher figures with incisions at 3 and 9 O'clock positions).10, 12 This has led to the search for new therapeutic methods such as the permanent stent in the external sphincter.
Urethral stents began to be used in 1988 for the treatment of bulbar urethra stenosis.16 Later on, these indications were extended to the treatment of benign prostatic hyperplasia17 and DSD.18
Our long-term aim is to assess the clinical and urodynamic results of intraurethral stents in a group of patients suffering from SCI.
Materials and methods
This paper is a descriptive retrospective study assessing 47 consecutive male patients who had an intraurethral stent placed between 1993 and 2002 at our hospital. All of them had bladder hyperreflexia with DSD. Our experience in this field of studying 24 patients for an average time of 15.4 months has already been reported.19 We now present a higher number of patients and a long-term follow-up.
Two different types of prostheses were used: Memotherm® and Urolume,® (No commercial party having a direct or indirect interest in the subject matter of this study has, or will confer, a benefit upon the authors or upon any organization with which the authors are associated.) both 3.5 cm long.
The stent was inserted with the same surgical technique and by the same surgeon. The technique consists of placing the device under epidural anaesthesia, guided by direct vision through a cystoscope to guarantee the adequate placement between the distal part of the veru montanum and the bulbar urethra, at least 5 mm below the external sphincter. Prophylactic antibiotics were used and a suprapubic catheter was placed during the postoperative period, which was withdrawn once the adequate functioning of the stent was verified.
The following parameters were compared before and after stenting:
-
1
Urodynamic parameters: forty-seven patients were assessed urodynamically before and after stenting. DSD was confirmed by electromyography. The urodynamic assessment was carried out with a multichannel device, using a 6 Ch double flow bladder catheter, a 10 Ch intrarectal catheter and superficial perineal electrodes. Physiological serum at room temperature was infused at a 30 ml/min rate. The urodynamic parameters assessed were leakage pressure (or, if not applicable, the maximum pressure achieved by the detrusor during the filling stage) and the volume of residual urine. In order to obtain the decrease in these parameters after the intervention, we calculated the difference between pressures or residues in each patient and then proceeded to calculate the average of these values. In patients who had previously used an indwelling catheter, we have recorded the residual urine after the procedure.
-
2
The presence or absence of symptomatic UTI. We only considered that there was a presence of UTI when the number of episodes was greater than two per year.
-
3
Autonomic dysreflexia, as assessed by the patient's subjective symptoms with regard to bladder manipulation, urinary infections, urine retentions, etc. Arterial pressure was not determined, except in isolated cases in which autonomic dysreflexia occurred at hospital.
-
4
The appearance of complications of the UUT, such as VUR, hydronephrosis, renal failure, renal lithiasis, etc.
-
5
Bladder management before and after surgery.
-
6
Prosthesis complications (migration, telescoping, stenosis, lithiasis, stent removal). The methods, definitions and units used are those recommended by the International Continence Society.20
Statistical analysis
-
All demographic data were presented as an average±standard deviation.
-
As for leakage pressure values and post-void residues, Wilcoxon's test was used to compare the decrease in these values for each patient and then to calculate the average. We used Pearson's correlation to establish a relation between post-void residual urine volume (PVR) decrease and follow-up time.
-
The decrease in UTI, dysreflexia, UUT involvement and VUR was assessed by using McNemar's test.
-
In all these cases, P<0.05 was considered statistically significant.
Results
Forty-seven patients aged between 32 and 80 (average age 52.7) years were included in the study. The mean time from the SCI up to the stenting was 103.8 months (11–312 months) and the average follow-up time from the implantation was 67 months (14–125 months). Sixty-eight per cent of the patients had cervical neurological level, 23% dorsal and 9% lumbar. According to the International Standards for Neurological and Functional Classification of Spinal Cord Injury,21 the injury was complete in 76.7% (ASIA A), and in 23.3% incomplete (9.3% ASIA B and 14% ASIA C). Sixteen patients (34%) had been submitted to a previous sphincterotomy, including one procedure in 12 (25.5%), two in three (6.4%) and three in one patient (2.1%).
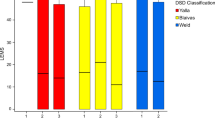
Detrusor pressure experienced an average decrease of 44.4 cm H2O (−20 to 142 cm H2O), which was statistically significant (P=0.0001), with the exception of a patient who had a negative evolution and experienced an increase of 20 cm H2O in detrusor pressure. Post-void residual volumes diminished 224 cm3 on average (0–350 cm3), which was also significant (P=0.001). This decrease was sustained in time, with no correlation with the number of months elapsed after stenting (Figure 1). Among those with previous indwelling catheter (19 patients), postoperative PVR residue was 108.86 cm3 (0–190 cm3); this figure was assessed in 14 patients because no data from the remaining five were available. The presence of UTI diminished by 25%, from 67.5 to 42.5% (P=0.031). The episodes of dysreflexia also decreased in a significant manner (P=0.039) from 35 to 16%. With regard to UUT complications, there was a decrease from 47 to 23% after the stent was implanted (P=0.013). The most frequent complication was VUR, which accounted for 65% of the UUT complications before placing the stent and 50% afterwards. Individually the number of patients suffering from VUR (in any degree, unilateral or bilateral) also experienced a significant decrease (P=0.013) from 33 to 11%.
The urinary management method changed substantially after placing the prosthesis (Figure 2). Before the intervention, the methods used were indwelling catheter (39%), intermittent catheterization (IC) (11%), collector (28%), collector associated to IC (20%) and a patient with suprapubic catheter. Out of the 19 patients who previously had an indwelling catheter after stenting, 16 (84%) were able to just manage with the collector. Of the patients without indwelling catheter, only two required this management method once the prosthesis was implanted.
Sixty-four per cent of the patients did not have prosthesis-related complications. The most frequent complication was migration, which occurred in 28% of the cases (13). The other complications we observed were: three encrustation from bladder lithiases (6%), one intraprosthetic calcification (2%) and seven stenoses (15%); five required transurethral resection of the hypertrophic tissue, and in the other two cases there was redundant tissue, but the problem was solved with an indwelling catheter for three months. A patient with calcification and another one with stenosis later required the removal of the device. In 12 of the 13 migrated prostheses, we conducted telescoping (in one case, we skipped this procedure owing to adequate bladder emptying). Nine of the telescoping procedures were conducted during the first year, including one case during surgery. Among the telescoped prostheses, seven evolved favourably and the other four cases had stenosis (in one case associated to lithiasis), of which two required the withdrawal of the device.
Overall, four urethral stents were withdrawn (8.5%); two of them owing to stent mobilization and bad evolution post-telescoping; and of the other two, one owing to intraprosthetic calcification and the other one owing to stenosis that was not solved by means of transurethral resection. In all cases, the removal was conducted without complications.
Of 47 studied prostheses, 29 were Memotherm (M) and 18 Urolume (U): 48% of M suffered from complications, opposite to 33% of U. The most frequent complication was migration (31% M, 33% U), followed by lithiasis (10% M, 11% U) and stenosis (7% M, none U). Four prostheses withdrawn were M.
Bladder neck obstruction has been included in other series as the most frequent complication of intraurethral stents.22 This condition is likely to have existed before, but could become evident after the resolution of DSD. In our series, before implanting the device, only two patients were treated with alpha-blockers and four had been submitted to cervicotomy owing to bladder neck obstruction. After surgery, 22 patients required alpha-blockers to control this condition, and in three cases (which were not treated previously) a bladder neck incision was performed during the device implantation because the neck was closed during the procedure.
Discussion
In some publications, the security and efficiency of the intraurethral stent has been compared with, as opposed to, sphincterotomy.11, 23 Conversely, several articles have been reported with the results of urethral prostheses in this type of patients, although in most cases patients were not monitored in the long term, and the number of subjects was inadequate.22, 24, 25, 26, 27 Only Chancellor's series, which studies 160 patients with an approximate follow-up period of 5 years,28 is comparable to ours in extension.
All studies yield results that are at least comparable to those obtained with external sphincterotomy, which has been the therapeutic ‘gold standard’ to date for this pathology. A very important advantage of stents to patients is the fact that they are potentially reversible, as they do not cause permanent damage in the sphincter structure. Furthermore, sphincterotomy has a higher percentage of complications stemming from the surgical technique which, despite having decreased in the last few years, are still higher than with less invasive modern techniques.
The removal of the device was a reason for concern in the past, which led to researchers investigating the likelihood of complications when removing the stent. For example, complications like bleeding or urethral trauma, especially on the sphincter, are rare although they also depend on the technique used. The prosthesis may be withdrawn without altering the function of the external sphincter and without leading to urethral stenosis, even after complete epithelialization and after an extended period of time since its implantation (>1 year).28, 29, 30, 31 In one of our patients, the device was withdrawn without any problems 4 years after the prosthesis was implanted. Because is a complex surgical process, a proper explantation technique is essential for a good outcome.31 Often, it is necessary to remove the stent, wire by wire, after resection of overlying U.32 In spite of it, we have removed permanent stents with minimal complications and no lasting consequences. Most withdrawals are conducted within the first year and are mainly owing to inappropriate implantation or displacement. This migration is generally minimal, but enough to compromise the efficiency of the stent. No predictive factors could be identified (Denys P et al27) even if on occasions, it has been attributed to inadvertent stent manipulation during digital bowel evacuation, scars secondary to prior sphincterotomies, etc, without concluding results. In our study, four prostheses were removed (8.5%), three owing to stenosis and one owing to lithiasis and recurrent infections. These percentages are similar to those in the literature.28 According to some reported articles, withdrawal is more frequent when prostheses are used in the treatment of DSD and benign prostatic hyperplasia, as compared with those used in urethral stenosis, even if the reason is unknown.33
As removal is difficult to perform, some authors have tried to find an alternative procedure, the temporary urethral stent (Memokath®). Although initial results appeared promising, almost all the stents required removal for complications of urinary infection, migration and encrustation.34 The ‘working life’ of a Memokath stent is 20–21 months.35, 36 Although we do not have experience with these devices, they might be used as a temporary measure in selected SCI patients: no history of recurrent UTI, need for manual bowel evacuation, potential to recover manual dexterity or if the patient wants to enter a fertility programme,36 etc.
Urethral stenosis is one of the possible prosthesis complications. We found a high percentage, almost 15%; in two cases, it was solved by means of indwelling catheter, and in five other cases, it was necessary to conduct an urethrotomy to remove the hypertrophic tissue. Stenosis was generally punctiform and was located at the joining between both prostheses (if there was prior telescoping) or on the distal edge, adjacent to the stent. There does not seem to be a correlation with the existence of a previous urethrotomy, as out of the seven patients, only two had been previously submitted to this intervention. There are published series in which it is claimed that an irregular stenosis of the stent lumen may be attributed to a response of the urothelium (a common phenomenon during the first month's postsurgery), which is generally solved in the first 6 months, achieving a relatively smooth lumen with a minimal tissue response.30, 37
A persistent obstruction after stenting may also result because of bladder neck obstruction. It has been attributed to neck dyssynergia and detrusor hypertrophy,38 and may become evident only after the treatment of DSD with the decrease in sphincter resistance. It may not be a strict failure of the stent or the sphincterotomy; however, it is a cause of persistent obstruction of the output flow,1 and in some of the series, it is the main complication after stent implantation.22 The moment when it arises varies depending on the series between 7 and 76 months, and it seems as if the frequency of this complication increases with follow-up time.25 On certain occasions, it responds to oral treatment with alpha-blockers, but at other times, a bladder neck incision is required to solve the obstruction. As bladder neck hypertrophy leads to recurrence even after surgery, it is necessary to conduct strict urodynamic monitoring after the procedure.25 This intervention may have a potential effect on the sexual function, which would alter the principle of reversibility of the prosthesis. However, the percentages of bladder neck obstruction reflected in the literature are similar for both techniques.23 In our series, 44% of the patients developed this complication de novo. Percentages vary a lot between series, varying between 7.7 and 71.4%.10, 11, 19, 22, 25, 28 There is an increase in this complication as the follow-up period is extended.
Comparing all the results with other series published to date, Chancellor's28 is the biggest and the one most similar to ours with regard to follow-up time. The results obtained in both cases are quite similar. In our series, there is a higher percentage of telescoping (25.5 vs 16.7% in his series) as well as of stenosis (10.6 vs 3.1%); a lower occurrence of stenosis (2.13 vs 7.5%) and also of prosthesis removal (8.5 vs 15%). Mobilization was similar in both series (27.7 and 28%), and the average follow-up period was approximately 5.5 years (67 vs 60 months). As for the global result, 79% of our patients had a good evolution, whereas in Chancellor's series this percentage rose to 84%. The most remarkable difference lies in the number of stenoses, which in our series was 10.6%, whereas in Chancellor's series it was only 3.1%.
It is difficult to compare our results with those of other series owing to either the small number of patients studied or the short follow-up period during which patients were monitored.
Although the percentage of complications was higher for Memotherm prostheses (48%) than for Urolume prostheses (33%), no statistically significant difference exists, probably because of the small number of patients in each group. The most common complication in both groups was the mobilization, with a similar percentage for both types (31–33%). All the devices removed (four) were Memotherm.
Presently, the urethral stent is an efficient treatment for DSD in patients suffering from SCI, achieving results that are comparable with those obtained with endoscopic sphincterotomy of the external sphincter. Nevertheless, present research is focused on finding an efficient pharmacological treatment without having to recur to invasive methods like those mentioned above. Thus, the most recent studies on nitric oxide or carbon monoxide are obtaining promising results, which could have important therapeutic implications in the future.1
Conclusions
From the present series, we may conclude that intraurethral stents are a good therapeutic option for those patients suffering from SCI with DSD who have been treated using indwelling catheter, including those submitted to previous sphincterotomy. It is easy to perform, with limited problems stemming from surgery and with results, which, despite not being perfect are, in our opinion, advantageous with regard to the other therapeutic options known to date. Furthermore, as it has the advantage of being a potentially reversible procedure, patients prefer this as opposed to more invasive therapies, such as sphincterotomy.
References
Reynard JM, Vass J, Sullivan ME, Mamas M . Sphincterotomy and the treatment of detrusor–sphincter dyssynergia: current status, future prospects. Spinal Cord 2003; 41: 1–11.
Frankel HL et al. Long-term survival in spinal cord injury: a fifty year investigation. Spinal Cord 1998; 36: 266–274.
Wein AJ . The role of external sphincterotomy for patients with a spinal cord lesion. J Urol 1998; 160 (Part 1): 961.
Shah NC, Foley SJ, Edhem I, Shah PJ . Use of Memokath temporary urethral stent in treatment of detrusor–sphincter dyssynergia. J Endourol 1997; 11: 485–488.
Madersbacher H, Wyndaele JJ, Igawa A, Chancellor MB, Chartier-Kastler EJ, Kovindha A . Conservative management in neuropathic urinary incontinence. In: Abrams P, Cardozo L, Khoury S, Wein A (eds). Incontinence. Proceedings of the 2nd International Consultation on Incontinence, Paris. July 1–3, 2001, 2nd edn. Health Publication Ltd.: Plymouth 2002 pp 697–755.
Siroky MB . Pathogenesis of bacteriuria and infection in the spinal cord injured patient. Am J Med 2002; 113: 67S–79S.
Perkash I . Donald munro lecture 2003 neurogenic bladder: past, present, and future. J Spinal Cord Med 2004; 27: 383–386.
Rivas DA, Chancellor MB, Staas Jr WE, Gomella LG . Contact neodymium: yttrium–aluminum–garnet laser ablation of the external sphincter in spinal cord injured men with detrusor sphincter dyssynergia. Urology 1995; 45: 1028–1031.
Vapnek JM, Couillard DR, Stone AR . Is sphincterotomy the best management of the spinal cord injured bladder? J Urol 1994; 151: 961–964.
Chancellor MB, Rivas DA, Abdill CK, Karasick S, Ehrlich SM, Staas WE . Prospective comparison of external sphincter balloon dilatation and prosthesis placement with external sphincterotomy in spinal cord injured men. Arch Phys Med Rehabil 1994; 75: 297–305.
Rivas DA, Chancellor MB, Bagley D . Prospective comparison of external sphincter prosthesis placement and external sphincterotomy in men with spinal cord injury. J Endourol 1994; 8: 89–93.
Yang CC, Mayo ME . External urethral sphincterotomy: long-term follow-up. Neurourol Urodyn 1995; 14: 25–31.
Juma S, Mostafavi M, Joseph A . Sphincterotomy: long-term complications and warning signs. Neurourol Urodyn 1995; 14: 33–41.
Ricottone AR, Pranikoff K, Steinmetz JR, Constantino G . Long-term follow-up of sphincterotomy in the treatment of autonomic dysreflexia. Neurourol Urodyn 1995; 14: 43–46.
Santiago JA . Sphincterotomy failure. J Am Paraplegia Soc 1993; 16: 164–168.
Milroy EJ, Chapple C, Eldin A, Wallsten H . A new treatment for urethral strictures: a permanently implanted urethral stent. J Urol 1989; 141: 1120–1122.
Williams G et al. Use of stents for treating obstruction of urinary outflow in patients unfit for surgery. BMJ 1989; 298: 1429.
Shaw PJ, Milroy EJ, Timoney AG, el Din A, Mitchell N . Permanent external striated sphincter stents in patients with spinal injuries. Br J Urol 1990; 66: 297–302.
Juan García FJ, Salvador S, Montoto A, Lion S, Balvis B, Rodríguez A et al. Intraurethral stent prosthesis in spinal cord injured patients with sphincter dyssynergia. Spinal Cord 1999; 37: 54–57.
Abrams P et al. Standardization of terminology of lower urinary tract function. The International Continence Society Committee on Standardization of Terminology. Scand J Urol Nephrol 1988; 114 (Suppl): 5–19.
American Spinal Injury Association. International Standards for Neurological Classification of Spinal Cord Injury, revised 2002; American Spinal Injury Association: Chicago, IL 2002.
Hamid R, Arya M, Patel HR, Shah PJ . The mesh wallstent in the treatment of detrusor external sphincter dyssynergia in men with spinal cord injury: a 12-year follow-up. BJU Int 2003; 91: 51–53.
Chancellor MB et al. Sphincteric stent versus external sphincterotomy in spinal cord injured men: prospective randomized multicenter trial. J Urol 1999; 161: 1893–1898.
Sauerwein D, Gross AJ, Kutzenberger J, Ringert RH . Wallstents in patients with detrusor–sphincter dyssynergia. J Urol 1995; 154 (Part 1): 495–497.
McFarlane IP, Foley SJ, Shah PJ . Long-term outcome of permanent urethral stents in the treatment of detrusor–sphincter dyssynergia. Br J of Urol 1996; 78: 729–732.
Vaidyanathan S, Soni BM, Oo T, Sett P, Hughes PL, Singh G . Long-term result of Memokath urethral sphincter stent in spinal cord injury patients. BMC Urol 2002; 2: 12.
Denys P, Thiry-Escudie I, Ayoub N, Even-Schneider A, Benyahya S, Chartier-Kastler E . Urethral stent for the treatment of detrusor–sphincter dyssynergia: evaluation of the clinical, urodynamic, endoscopic and radiological efficacy after more than 1 year. J Urol 2004; 172: 605–607.
Chancellor MB, Gajewski J, Ackman CF, Appell RA, Bennett J, Binard J et al. Long-term followup of the North American multicenter urolume trial for the treatment of external detrusor–sphincter dyssynergia. J Urol 1999; 161: 1545–1550.
Chancellor MB et al. Reversible clinical outcome after sphincter stent removal. J Urol 1996; 155: 1992–1994.
Parikh AM, Milroy EJ . Precautions and complications in the use of the urolume wallstent. Eur Urol 1995; 27: 1–7.
Gajewski JB, Chancellor MB, Aceman CF, Appell RA, Bennett J, Binard J et al. Removal of urolume endoprosthesis: experience of the North American Study Group for detrusor–sphicnter dyssynergia application. J Urol 2000; 163: 773–776.
Nambirajan T, Woolsey S, Mahendra V, Stone AR, Walsh IK . Urethral stents for detrusor–sphincter dyssynergia. BJU Int 2005; 95: 350–353.
Shah DK, Kapoor R, Badlani GH . North American Study Group. Experience with urethral stent explantation. J Urol 2003; 169: 1398–1400.
Low AI, McRae PJ . Use of the Memokath for detrusor–sphincter dyssynergia after spinal cord injury – a cautionary tale. Spinal Cord 1998; 36: 39–44.
Hamid R, Arya M, Wood S, Patel HRH, Shah PJR . The use of the Memokath™ stent in the treatment of detrusor sphincter dyssynergia in spinal cord injury patients: a single-centre seven-year experience. Eur Urol 2003; 43: 539–543.
Metha SS, Tophill PR . Memokath® stents for the treatment of detrusor sphincter dyssynergia (DSD) in men with spinal cord injury: The Princess Royal Spinal Injuries Unit 10-years experience. Spinal Cord 2006; 44: 1–6.
Chancellor MB et al. Placement of a wire mesh prosthesis in the external sphincter of men with spinal cord injuries. Radiology 1993; 187: 551–555.
McInerney PD, Vanner TF, Harris SA, Stephenson TP . Permanent urethral stents for detrusor sphincter dyssynergia. Br J Urol 1991; 67: 291–294.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Seoane-Rodríguez, S., Sánchez R-Losada, J., Montoto-Marqués, A. et al. Long-term follow-up study of intraurethral stents in spinal cord injured patients with detrusor-sphincter dyssynergia. Spinal Cord 45, 621–626 (2007). https://doi.org/10.1038/sj.sc.3102011
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.sc.3102011
Keywords
This article is cited by
-
Contemporary Treatment of Detrusor Sphincter Dyssynergia: a Systematic Review
Current Bladder Dysfunction Reports (2018)
-
US Experience With Bladder Management Following Spinal Cord Injury
Current Bladder Dysfunction Reports (2010)