Abstract
Study design:
Two case reports.
Objective:
To describe two unusual cases of deep diving followed by cerebro-spinal decompression sickness (DCS).
Setting:
Midlands Centre for Spinal Injuries, England.
Methods:
Observation of the outcome of two different cases of cerebro-spinal DCS, who have received two different modalities of treatment.
Results:
The first patient's symptoms developed after he surfaced, he was treated according to the US Navy treatment table 6. He also received steroids for almost 3 weeks. His MRI of the brain and spinal cord, which was performed within 24 h of injury did not show any abnormality, while a repeat MRI 3 weeks later revealed abnormal signals in the brain and spinal cord. The second patient's symptoms started before he surfaced, he was treated with Comex 30 treatment table for 14 days and received no steroids, his MRI was performed 3 days after the injury showed high signals in the brain and spinal cord.
Conclusion:
Both divers developed cerebro-spinal dysfunction. They had encephalopathy (manifested by loss of consciousness), which indicates bilateral cerebral dysfunction. DCS can occur even when dives are conducted according to the procedures described by the US Navy. The use of high-dose steroids has not been formally tested in DCS; their use is controversial.
Similar content being viewed by others
Introduction
Decompression sickness (DCS) is a systemic disease that can result in severe and disabling neurological consequences. DCS was first recorded in 1841 when deep-sea divers began to experience a strange inability to bend their joints. However, the reason for the condition was not fully understood until 1878, when Paul Bert published his theory that the cause was the formation of nitrogen bubbles within the body. He also correctly stated that it was possible to avoid their harmful effects by ascending to the surface gradually – and that hyperbaric chambers worked, in part, because they decreased the size of bubbles.1
DCS can present in a number of ways. Divers may present with concomitant disease process with nonspecific symptoms making diagnosis sometimes difficult, time consuming, and can be challenging.
In the UK, there are almost 300 cases of bends every year.1 The spinal cord is the most common site for Type II DCS. One percent of dives result in DCS.2 It is a clinical syndrome caused by a decrease in ambient pressure. As a diver descends, the increasing ambient pressure causes inert gases to dissolve in tissues. Ascents may result in liberation and formation into the tissues or blood of an inert gas bubbles leading to the clinical manifestations of decompression illness.
-
The actual mechanism of bubble formation is when the diver suddenly rises to sea levels, the pressure on the outside of the body becomes 760 mmHg, where the pressure inside the body is about 4000 mmHg (this is at sea level. However, the pressure changes would depend mainly on the depth of the dive) which is far greater than the outside of the body. Therefore, the gases can escape from the dissolved state and form bubbles in the various body tissues including the circulation where at first they plug the smallest blood vessels, but as they coalesce progressively larger vessels are affected.3
-
Based on the clinical manifestations, the generally accepted classification of DCS is Type I or Type II.
-
Type I includes joint pain, skin rash, and marbling or localized oedema. Type II has more serious symptoms dominated by injury to the central nervous system mainly the spinal cord. The spinal cord is affected in 20–50% of cases who develop Type II DCS, and is frequently resistant to recompression therapy.3, 4 Symptoms mimic spinal cord trauma, low back pain may start within a few minutes to hours after the dive and may progress to paresis, paralysis and loss of sphincter control.4
Case I
A 35-year-old male, lorry driver and recreational diver. Normally fit and well apart from mild hypertension which was diagnosed a few weeks before his last dive. He descended down to 37 m, total bottom time was 25 min, and he spent 4 min to ascend to 17 m when he stopped for 2 min. It took him a further 7 min to ascend to another 17 m when he again stopped for 2 min; he surfaced safely and swam back to shore 25 m. The water temperature was around 25°, the breathing gas was air. The diving conditions were described as very relaxed and well within the safety limits. A few minutes later he experienced abnormal sensation down both lower limbs following which he lost his consciousness for approximately 20 min. (He denies any chest pain, pulmonary symptoms, back or abdominal pain or abnormality in the upper limbs.) His friend who witnessed him confirmed that the patient had no seizures. He woke up in the accident and emergency department when he was still able to move all four limbs. However, the abnormal sensation in both lower limbs was still present.
He was diagnosed as having DCS, and treated immediately with 100% oxygen, intravenous fluids and 35 mg of intravenous dexamethasone as a bolus dose followed by 4 mg of oral dexamethasone six hourly for the following 14 days. At 1 h after the accident he was put in the decompression chamber (United States Navy treatment table 6 – using oxygen). He was pressurized down to 50 m, he spent five and a half hours in the chamber, he was able to move all four limbs when examined half an hour before he left the chamber. He was receiving 100% oxygen all the time. However, the last 40 min he was pressurized only to 9 m and he received only 40% oxygen.
Only after he came out of the chamber did he realize that he could not move his legs, (the patient could not give an accurate timing) and he became paraplegic (T12 Frankel grade B, both knee and ankle reflexes reported to be present). He also lost his bladder and bowel control. An echocardiogram was performed and was found normal.
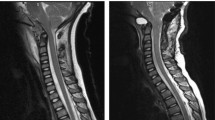
An MRI scan (signa 1.5 T) of the brain and whole spinal cord was undertaken within 24 h of the injury, which failed to show any abnormalities. MRI was again repeated 3 weeks after the injury. This showed an area of abnormal high signal within the posterior aspect of the cord from C3 level down to C4/5 disc space (Figure 1). The MRI brain revealed an area of high signal lying high within the left cerebral hemisphere just supero-lateral to the left ventricle (Figure 2).
(a) The image attached depicts a saggital T2, STIR sequence MRI scan, taken 3 weeks after the accident (no axial views were taken at this time) showed an area of abnormally high signal within the posterior aspect of the spinal cord from C3 to C4–C5 disc space. (b) This image depicts a saggital T2 weighted sequence MRI scan, taken 7 weeks after the accident, there is again a zone of faint increased signal within the posterior aspect of the cord just extending from C3 to C5, but principally being present at the level of C4. (c) This is an axial T2 weighted sequence MRI scan again 7 weeks after the accident where the high signal appears to be located with two almost punctuate-like foci in the posterior aspect of the cord at the level of C4
At 3 weeks after the injury, he started to show some flickers of movements in hip adductors, knee flexors and ankle dorsiflexors. After 4 weeks, hips and knee movements were 3/5; ankle movements were 1/5 bilaterally.
He was reviewed in the outpatient clinic 20 months after the injury. His hip and knee motor power were 3-4/5, ankle movements were 3/5, proprioception was still impaired. He was able to walk indoors for approximately 100 yards using two crutches. He had normal sensation of desire to defecate, and of defecation, and was able to feel and control micturition for a very short period of time.
Case II
A 56-year-old male self-employed (financial adviser), recreational diver, fit and well. He descended to 57 m. The temperature was 4° at the bottom and approximately 15° during the rest of the dive. He went through a tunnel on the same depth, from that moment he could not remember any thing until 3 days after the accident. He was breathing air. According to his dive partner, the valve device froze in an open position and was continuously pouring air out, and he started to use the air regulator, he was behaving normally during his ascent BUT with NO stops.
On arrival at the platform, he was told; he lost consciousness for 10–15 min and had generalized seizures. After he woke up he was not able to move any of his four limbs.
He was diagnosed as having DCS and was immediately given 100% oxygen, intravenous fluids. After 20 min he was transferred to the decompression chamber (Comex treatment table CX-30, using 50% helium in addition to 50% oxygen, ie, heliox 50/50). He was put in the chamber for 5 h on the first day, then daily for 1 h for 2 weeks. On the third day after the accident, he could only recall incidents that happened 3 days after the accident. He was told that he was tetraplegic from the beginning. He had grade 0/5 in both lower limbs, only flickers of movements on both hands and grade 1-2/5 on both elbows. His sensation was abnormal in both upper and lower limbs below C6. Both ankle and knee reflexes were absent. He was doubly incontinent.
At 10 days after the accident his hand function had improved and became 3/5 and he started to show some flickers in both lower limbs. At 6 months after the accident, his upper limb neurology was still 3/5, lower limbs 2-3/5. He continued to have abnormal proprioception. He remained doubly incontinent. An echocardiogram was not performed in this case.
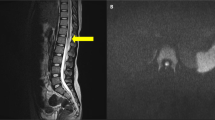
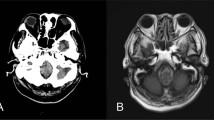
An initial MRI scan of the brain obtained 3 days after the accident revealed an abnormally high signal in the white matter (Figure 3). This had resolved on repeat MRI performed 8 weeks after the injury. Also, an MRI of the cervical spine performed 3 days after the accident showed an abnormally high signal at C3–4 level (Figure 4), which then disappeared on repeat scan 10 weeks later.
(a) This is a saggital T2 weighted MRI scan of the cervical spine taken 3 days postinjury. It depicts an abnormal high signal at the C3–C4 of the spinal cord. There is also a disc herniation at C5–C6 disc space but with No cord signal (no axial images were taken at this stage). (b) This is a T2 axial MRI image of the cervical spine taken 10 weeks after the accident. It depicts a small right-sided disc herniation at C5–C6 level, some alterations in signal are present in the C5–C6 vertebrae, however; there is no evidence of cord lesion at this level
He was reviewed in the outpatient clinic 15 months after the accident; his upper limb function was still 3/5, while hips and knees were 2-3/5, and ankle movements were only 1-2/5. He was able to walk only very few steps indoors using the ankle foot orthosis and with the help of a walking frame. He was emptying his bowel by digital stimulation and straining and his bladder with a suprapubic catheter.
Discussion
The incidence of DCS in the diving community is generally low ranging from 0.013 to 1.25%.5 The spinal cord is affected in 3–4% of cases. The lower thoracic region is a commonly affected area of the spinal cord followed by the lumbar then cervical. The spinal cord white matter is particularly vulnerable to bubble formation because nitrogen is highly soluble in myelin and it has poor collateral blood supply.2
In the US, during 1995, 590 cases of DCS were analysed, of these 27.3% were Type I DCS (joint pain and skin manifestation only), and 64.9% were Type II where the spinal cord was involved, the remaining were arterial gas embolism with cerebral symptoms.6
The pathphysiology3 of decompression illness is still debatable; there are several proposed mechanisms to explain the actual patho-physiological process.
The current leading theory suggests myelopathy results from congestion of the spinal epidural venous plexus (batson's plexus), with subsequent spinal cord ischaemia, also gas bubbles in the venous bed may accelerate coagulation and activate complement leading to more complement venous obstruction.
The second theory is the autochthonous theory, which suggests decompression causes gas bubbles to nucleate within the tissues themselves. This may also occur as a result of decreased blood flow due to venous congestion. Gas bubbles may also act as a space occupying lesion leading to further disruption of blood flow and damage to the myelin sheath. The high-fat content of the myelin, together with the high solubility of nitrogen in fat explains the vulnerability to injury of the spinal cord.
The third theory which seems to have fallen out of favour, suggests gas bubbles released from tissues coalesce in the arterial system and obstruct the arterial circulation. However, this mechanism is inconsistent with the high rate of spinal cord involvement compared with the cerebral involvement seen in decompression illness.
The Diver's Alert Network (DAN) has stated; ‘The supposition of any damage to the brain rests on the occurrence of so called silent bubbles occurring in the blood or brain and spinal cord.’ That such bubbles do exist has been well documented by Doppler technology in the blood and tissues of animal's spinal cords. Whether or not, however, these silent bubbles are the cause of changes in the brain is unproved.7
The spinal cord is the most common site for Type II DCS. However, it is uncommon to have both spinal cord as well as cerebral injury together.
Both patients developed higher cerebral function impairment (Encephalopathy) early on during the course of their illness manifested by loss of consciousness, which indicates bilateral cerebral dysfunction or brain stem (reticular activating system) involvement. Interestingly, MRI scan of the first patient within the first 24 h failed to show any abnormality, however, repeat scans 3 weeks later did reveal abnormally high signals in both the brain and the spinal cord. The second patient had MRI scans 3 days after the injury and did show abnormally high signal in both the brain and the spinal cord.
The neurological presentation of DCS and arterial embolism in sports divers is well documented.8 Delays in developing symptoms for up to 24 h from a dive can present diagnostic difficulties to the clinician. Our first patient reports a relaxed dive and managed to swim a distance of 25 m to the shore. His first symptoms were of abnormal sensation in the lower limbs followed by loss of consciousness. He did not develop any other symptoms and was actually observed to move his legs in the decompression chamber half an hour before he left the chamber. It was not until he came out of the chamber that he realized he was paraplegic.
Patients with spinal cord injury (SCI) due to DCS have a much more favourable prognosis than those with SCI due to other causes.9
Research by the Divers Alert Network (DAN) showed that while 75% of divers with DCS make a complete recovery after treatment in a hyperbaric chamber, the remaining 25% are less fortunate. Of the divers with DCS, 20% are likely to make a more gradual recovery, possibly sustaining some long-term damage, and 5% have injuries which do not respond to hyperbaric treatment. It is these divers who may benefit from any progress in regenerative spinal cord treatment.10
According to DAN data of the US Navy treatment table 6 was used for initial treatment in approximately 75% of sport divers with DCS. For persistent neurological symptoms, treatment should be continued until no significant improvement occurs on two consecutive treatments. Most divers receive between 5 and 10 treatments; however, a minority, with severe residual neurological injury may require 15–20 treatments. The first patient was in the decompression chamber only once and started to show flickers of movement about 3 weeks later. The second patient continued to receive an hour of decompression in the chamber daily for 2 weeks. He started to exhibit flickers of recovery 10 days after the incident. It is difficult to suggest from these two cases that had the first patient underwent further decompression he would have started recovering earlier.
The use of high-dose steroids has not been formally tested in DCS; their use is still controversial (only 2.3% of divers treated with steroids in 1998).2 Our first patient received steroids for 3 weeks while our second patient did not. Steroids do not seem to have made a difference to the outcome at 15 months for the first patient and 20 months for the second patient.
Long-term effects of deep diving include dysbaric osteonecrosis (a form of aseptic bone necrosis), decreased pulmonary function due to airway narrowing hearing loss, liver changes, cognitive impairment and spinal cord dysfunction. Fortunately, other than spinal cord dysfunction neither of our patients developed other long-term effects.
Typically in our patients' nerve tissues in the spinal cord was starved of oxygen because the small capillaries that carry blood to the tissues were probably damaged. Some blood flow must, however, have been restored within hours, for the nerve tissue in the spinal cord to escape death from complete lack of oxygen and complete paralysis.11
Conclusion
Although injury is less severe when safe diving practices are used, no level of caution can completely eliminate the risk of DCS after an underwater dive. The symptoms related to DCS can be delayed in their appearance as demonstrated by our first patient. Our two patients were rather unlucky to present with both cerebral and spinal involvement in the acute stage and not to have fully recovered, as would the majority of patients following decompression. They are, however, relatively lucky in that they have partially recovered neurologically without sustaining long-term cerebral or other sequelae.
The role of steroids has yet to be established in DCS.
References
Gordon B . Actor Alki David talks about getting the bends. In: Cyber Diver News Network 2004 May 24 [Online] [access May 2005]. Available from URL http://www.cdnn.info/safety/s040524/s040524.html.
Barratt DM, Harch PG, Van Meter K . Decompression illness in divers: a review of the literature. Neurologist 2002; 8: 186–202.
Kimbro T, Tom T, Neuman T . A case of spinal cord decompression sickness presenting as partial Brown-Sequard syndrome. Neurology 1997; 48: 1454–1456.
Eden J . Decompression sickness. In: eMed Private Medical Services 2004 [Online] [access July 2005]. Available from URL http://www.e-med.co.uk/diving/decompression_sickness.php.
Petri NM, Andric D . Differential diagnostic problems of decompression sickness – examples from specialist physicians' practices in diving medicine. Arch Med Res 2003; 34: 26–30.
Dulley SA . Decompression sickness. In: eMedicine 2002 [Online] [access 2004 July]. Available from URL http://www.emedicine.com/emerg/topic121.htm.
Campbell ES . Long term effects of diving. In: Medscape Orthopaedics & Sports Medicine e-Journal 1998 2 (5) [Online] [access April 2005]. Available from URL http://www.medscape.com/viewarticle/408486_12.
Dick A, Massey E . Neurologic presentation of decompression sickness and arterial embolism in sport divers. Neurology 1985; 35: 667–671.
Injury during diving or work compressed air. In: Beers MH, Berkow R (eds). Merck Manual of Diagnosis and Therapy. 17th edn. Merck Research Laboratories: New Jersey 1999 Chapter 285. [Online] [access April 2005]. Available from URL http://www.merck.com/mrkshared/mmanual/section20/chapter285/285b.jsp.
Spinal injuries research gives new hope to bends victims. In: Divernet Magazine 2004 Feb 4 [Online] [access February 2004]. Available from URL http://www.diverner.com/new/stories/spine040204.shtml.
High pressure chambers could prevent paralysis after injury. In: Science Daily 1998 May 14 [Online] [access April 2005]. Available from URL http://www.sciencedaily.com/releases/1998/05/980514080559.htm.
Acknowledgements
Special thanks to Siobahn Whitby for her critical comments on the earlier version of the manuscript. Also, we gratefully acknowledge the valuable assistance received from Hiyam Elhenshiri during the preparation of this article.
Author information
Authors and Affiliations
Additional information
Patient Confidentiality: I can confirm that Consent from both patients was obtained for the purpose of this paper
Rights and permissions
About this article
Cite this article
Jallul, S., Osman, A. & El-Masry, W. Cerebro-spinal decompression sickness: report of two cases. Spinal Cord 45, 116–120 (2007). https://doi.org/10.1038/sj.sc.3101923
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.sc.3101923
Keywords
This article is cited by
-
Novel Conductive Carbon Black and Polydimethlysiloxane ECG Electrode: A Comparison with Commercial Electrodes in Fresh, Chlorinated, and Salt Water
Annals of Biomedical Engineering (2016)
-
Simulated dive in rats lead to acute changes in cerebral blood flow on MRI, but no cerebral injuries to grey or white matter
European Journal of Applied Physiology (2013)