Abstract
Study design:
This is a case report of a patient with hypertrophy of the posterior longitudinal ligament (HPLL) in the thoracic spine. This patient was followed for 10 years after surgery.
Objectives:
The purpose of this study was to report the long-term outcome of HPLL in the thoracic spine.
Setting:
Department of orthopedic surgery, Hiroshima Red Cross and Atomic-bomb Survivors Hospital, Hiroshima, Japan.
Methods:
A 58-year-old-woman with thoracic HPLL was reported. Magnetic resonance image (MRI) and computed tomography (CT) showed the expanded spinal cord compression from Th4 to Th12 due to HPLL. Anterior decompression and fusion (Th10–12) was performed. Histological findings of the surgical specimens showed thickening of the posterior longitudinal ligament with proliferation of chondroid tissue. The clinical outcome and the radiological findings (CT and MRI) were evaluated 10 years after surgery.
Results:
The patient was asymptomatic postoperatively. However, the subsequent CT examination revealed ossification of the previously hypertrophied posterior longitudinal ligament.
Conclusions:
HPLL in the thoracic spine is a rare pathological condition causing myelopathy. The results of this study support the hypothesis that HPLL is one of the prodromal conditions of HPLL.
Similar content being viewed by others
Introduction
Although ossification of the posterior longitudinal ligament (OPLL) is a well-known disorder that leads to myelopathy, hypertrophy of the posterior longitudinal ligament (HPLL) is a rare pathological condition. HPLL is defined by thickening of the PLL that compresses the dural tube. The differential diagnosis between these disorders should be made based on the existence of ossification either on the X-rays or the histological findings. The specimen from the OPLL lesion showed the lamella bone formation, which is not detected in the HPLL lesion. Some reports1, 2, 3, 4 have suggested that HPLL is a prodrome of OPLL. However, there is no universal consensus regarding the relation between OPLL and HPLL. Here, a case of HPLL is reported in the thoracic spine; the patient was surgically treated and followed for a 10-year-duration.
Case report
A 58-year-old female was admitted with a 2-month-history of the spontaneous onset of progressive lower extremity paresthesias and progressive gait disturbance. She exhibited a spastic paraparesis characterized by motor weakness of the right lower extremity and sensory loss below the L1 level bilaterally. Bilateral hyper-reflexia and Babinski responses were observed.
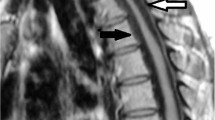
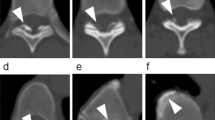
A plain radiographic examination showed mild spondylotic changes, and no abnormal ossification was realized. The magnetic resonance image (MRI) demonstrated extensive ventral compression of the dural tube attributed to a low-intensity signal band observed on T1 and T2-weighted images. It extended from the T4 to the T12 levels, with maximal compression being observed opposite the T10–T12 vertebrae (Figure 1a). Myelo-computed tomography (CT) examination confirmed extensive anterior compression by hypertrophied PLL as these levels (Figure 2a, b).
Serial T2-weighted sagittal MRI. (a) The compression of the spinal cord was showed at the level from Th4 to Th12 before surgery. (b) The compression on the spinal cord by the yellow ligament at the level of Th10–11 remained at 4-month after surgery. (c) The intensity and the size of the HPLL were not significantly changed at 10 years after surgery
As maximal compression was demonstrated at the T10–12 levels, the patient underwent ventral decompression and fusion in this region. Hypertrophied PLL was observed intraoperatively and lateral pathologically confirmed (Figure 3).
Postoperatively, the patient's symptoms and signs gradually regressed. She was asymptomatic able to play table tennis, 10 years postoperatively. The plain X-rays demonstrated good stabilization from T10–T12 levels. However, a high-density area was newly observed within residual HPLL on the recent CT study, indicating progressive ossification had occurred within residual HPLL (Figure 2c, d). An MR study had originally been performed 4 months and 10 years postoperatively; the size of the lesion now noted to be ossified on the CT study had not changed (Figure 1b, c).
Discussion
The relation between HPLL and OPLL is still controversial and there is no universal consensus as to whether HPLL evolves into OPLL. Epstein1 demonstrated that hypertrophy of the PLL without ossification could be seen in patients with OPLL at other levels in the cervical spine and concluded that OPLL and HPLL were related entities, progressive ossification within HPLL leading toward OPLL. Mizuno et al3 considered that HPLL might be a result of the process of PLL degeneration without ossification, which is caused by the metaplasia from collagenous fibers of ligament to chondrocytes. Then, HPLL might be replaced by compact lamellar bone, as recognized in OPLL lesion, when HPLL has some potential of systematic or genetic factors to induce secondary ossification in the PLL.
On the other hand, Nakamitsu et al,5 Matsumoto et al6 and Yoshizawa et al7 reported a few cases that showed no appearance of ossification on the histological findings within HPLL, and they concluded that HPLL was not a prodrome of OPLL, but a primary hypertrophy of the PLL.
In this study, the calcification or ossification change was realized in the HPLL lesion of our case 10 years after surgery. As no histological examination was performed, it is not clear that the high-density lesion evaluated by CT represent the actual ossification. However, it is reasonable to suppose that the ossification change had gradually advanced in the HPLL lesion during a long period of time. We consider that HPLL might be a prodromal condition of OPLL, and the strength of personal systematic or genetic factors might set a time of ossification change in HPLL lesion. The cases reported by Yoshizawa,7 Nakamitsu5 and Matsumoto6 might demonstrate the ossification change of HPLL, if the follow-up could be performed for more long time.
Several cases of HPLL have been reported,1, 2, 3, 4, 5, 6, 7, 8 however, the long-term follow-up study is rare. This is the first report of a case, in which the ossification change was confirmed in thoracic HPLL lesion by CT examination after a long-time follow-up. We speculated that HPLL might be one of the prodromal conditions of OPLL. Further follow-up of this patient is necessary to understand the natural course of HPLL and the relation between OPLL and HPLL.
References
Epstein NE . Advanced cervical spondylosis with ossification of the posterior longitudinal ligament and resultant neurologic quelae. J Spinal Disord 1996; 9: 477–484.
Kondo S et al. Hypertrophy of the posterior longitudinal ligament is a prodromal condition to ossification. Spine 2001; 26: 110–114.
Mizuno J, Nakagawa H, Hashizume Y . Analysis of hypertrophy of the posterior longitudinal ligament of the cervical spine, on the basis of clinical and experimental studies. Neurosurg 2001; 49: 1091–1098.
Toguchi A et al. An operative case of hypertrophy of the posterior longitudinal ligament in thoracic spine. J East Japan Association of Orthopedic Traumatology 1991; 3: 524–528 (in Japanese).
Nakamitsu K et al. Thoracic myelopathy caused by hypertrophy of posterior longitudinal ligament: a case report. Rinshoseikeigeka 1990; 25: 1087–1090 (in Japanese).
Matsumoto T et al. Lumbar canal stenosis caused by hypertrophy of the posterior longitudinal ligament: case report. Spine 2001; 26: E576–E579.
Yoshizawa T et al. Cervical myelopathy due to Hypertrophy of the posterior longitudinal ligament (HPLL): a case report. Rinshoseikeigeka 1991; 31: 720–724 (in Japanese).
Nozawa S et al. Sudden onset of paraparesis caused by hypertrophy of the thoracic posterior longitudinal ligament. Spinal Cord 2003; 41: 53–55.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ikuta, K., Arima, J., Sasaki, K. et al. Hypertrophy of the posterior longitudinal ligament in the thoracic spine. Spinal Cord 44, 200–202 (2006). https://doi.org/10.1038/sj.sc.3101812
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.sc.3101812
Keywords
This article is cited by
-
Hypertrophied posterior longitudinal ligament and ligamentum flavum causing myelopathy: a case report and literature review
Spinal Cord Series and Cases (2023)