Abstract
Study design:
Four-month longitudinal within-subject exercise training study.
Objective:
Although body-weight supported treadmill training (BWSTT) has not shown promise as a means of improving ambulation in individuals with motor-complete spinal cord injury (SCI), it may still improve cardiovascular health and function in this population. The purpose of this study was to (i) investigate the effects of BWSTT on peripheral muscular and elastic artery dimension and function and measures of heart rate variability (HRV) and blood pressure variability (BPV) in individuals with motor-complete SCI, and (ii) to make a preliminary examination of what factors may predict favourable cardiovascular outcomes following BWSTT in this population.
Setting:
Centre for Health Promotion and Rehabilitation, McMaster University, Hamilton, Ontario, Canada.
Methods:
Six individuals (four male, two female; age 37.7±15.4 years) with chronic SCI (C4-T12; ASIA A-B; 7.6±9.4 years post-injury) were included in the present investigation. Doppler ultrasound was used to determine femoral (exercising; muscular), carotid (elastic) and brachial (nonexercising control; muscular) artery dimension and function before and after 4 months of BWSTT. Continuous heart rate and blood pressure were also recorded before and after 4-months of BWSTT to determine frequency domain measures of HRV and BPV; clinically valuable indices of neurocardiac and neurovascular control, respectively.
Results:
Two-way ANOVA (vessel × time) revealed no exercise-induced change in femoral or carotid artery cross-sectional area, blood flow or resistance and no change in carotid artery compliance following the 4 months of BWSTT compared to the nonexercising control brachial artery. However, there was a significant exercise-induced increase in femoral artery compliance. There were no exercise-induced changes in HRV or BPV when all participants were considered together. However, the results suggest that the subgroup of individuals who had a substantial heart rate response to BWSTT (n=3), experienced exercise-training induced changes in HRV reflective of a relative shift toward cardiac vagal predominance and reductions in BPV.
Conclusions:
BWSTT may cause an increase in femoral artery compliance in individuals with motor-complete SCI and therefore, should be encouraged as a means of improving cardiovascular health in this population. BWSTT may also cause modest improvements in measures of HRV and BPV in a select subgroup of individuals who respond to ambulation with moderate to large increases in HR. In the present study, factors associated with a substantial HR response to BWSTT were a propensity to orthostatic intolerance and muscular spasticity.
Similar content being viewed by others
Introduction
Individuals with spinal cord injury (SCI) are prone to severe cardiovascular dysfunction and an increased risk of mortality from various cardiovascular diseases.1 Further, the risk of cardiovascular mortality appears to be heightened in those with more severe or complete SCI.1, 2 Although the underlying mechanisms responsible for the increased risk are not precisely understood, the loss of mobility and reduced activity levels that accompany severe SCI certainly contribute.
Unfortunately, complete muscular paralysis limits the options for exercise rehabilitation, and the current exercise strategies for individuals with complete SCI have various shortcomings that may reduce their effectiveness from a cardiovascular perspective. Although arm ergometry may provide a significant cardiovascular challenge for those with adequate arm function, it does not target the vessels of lower limbs; which contribute importantly to the increased cardiovascular risk in individuals with SCI.3, 4 In addition, although functional electrically stimulated (FES) exercise has shown great promise as a cardiovascular stimulus,5, 6, 7 it carries the risks of burns to the skin, autonomic dysreflexia and bone fracture, and further, stimulated contractions may not be evoked in those with lower motor neuron injuries.8 The cardiovascular benefit of FES exercise may also be limited as individuals with SCI are more prone to muscle fatigue,9, 10, 11 and may only perform such exercise for relatively short durations.11
In the last decade, body-weight supported treadmill training (BWSTT) has shown promise as a means of enhancing gait recovery in SCI individuals with partially spared motor function,12, 13, 14, 15, 16 but not in those with motor-complete SCI. The latter group have therefore been generally excluded from this therapy.17, 18, 19 With respect to cardiovascular benefit, however, BWSTT may offer distinct advantages over other exercise interventions, even in individuals with motor-complete SCI. First, unlike arm ergometry, BWSTT is an upright exercise that specifically targets the lower limbs. Second, because of the passive nature of the exercise and the body-weight support, this type of training can be performed for long durations without fatigue. Finally, BWSTT may be performed in individuals with upper or lower motor neuron injuries and without many of the inherent risks associated with FES exercise.
From a cardiovascular perspective, the value of BWSTT may be best evaluated by measures of vascular function as well as by indices of neural control of cardiovascular regulation, as both have clinical value and may be adversely altered after SCI. Regarding the former, recent research has shown that individuals with complete SCI exhibit peripheral vascular changes that likely contribute to their increased cardiovascular risk. Specifically, studies have found the luminal diameter to be significantly reduced20 and the vascular resistance to be significantly increased6, 21 in the femoral artery of individuals with complete SCI, and accordingly, resting femoral artery blood flow may be reduced by approximately 50% compared to that of able-bodied individuals.4 Clinically, this reduction in blood flow has been hypothesized to contribute to thrombus formation,3, 4 delayed wound healing21 and an increased risk of pressure sore formation.21 Individuals with SCI have also been shown to suffer from a reduced compliance of the femoral artery.20 Arterial compliance, or the ability of an artery to expand and recoil in response to changes in intravascular pressure, is particularly important as it allows the vessel to accommodate pulsatile increases in blood volume and thus confers a protective effect against vessel wall damage.22 Clinically, reductions in arterial compliance are thought to be a contributing factor to vascular damage, and the associated risks of thrombosis, myocardial infarction and stroke.23
With respect to neural regulation, power spectral analysis of heart rate variability (HRV) and blood pressure variability (BPV) have become commonly used, non-invasive methods used to quantify the autonomic control of the cardiovascular system.24, 25, 26, 27 Regarding HRV, successive R–R intervals obtained from electrocardiogram (ECG) recordings have been shown to oscillate around two main frequencies. The high-frequency oscillation, centred around 0.25 Hz (HF; 0.15–0.40 Hz) corresponds to parasympathetic outflow to the heart via the vagus nerve,28 while the low-frequency oscillation, centred around 0.1 Hz (LF; 0.04–0.15) has been shown to correspond to both the sympathetic and parasympathetic outflow to the heart, although it is much more indicative of the former.28, 29 Thus, the LF:HF ratio of HRV has become an accepted measure of cardiac sympathovagal balance. Similarly, the LF oscillation of systolic BPV (LFSBP; 0.04–0.15 Hz), also referred to as Mayer waves, has been shown to correspond to neurovascular control via the sympathetic nerves.26, 27 Measures of HRV and BPV have been found to have significant clinical value, as relatively decreased cardiac vagal predominance has been associated with an increased risk of cardiovascular mortality,30, 31 and increased BPV and enhanced Mayer waves are associated with end-organ damage.32, 33 Previous work from our lab has shown positive changes in HRV and BPV following BWSTT in individuals with motor-incomplete SCI,34 however, it is unknown if individuals with motor-complete SCI may experience the same benefit, due to their more passive participation in the exercise. In addition, previous work from our lab has shown measures of HRV and BPV to be reproducible in individuals with spinal cord injury (Ditor et al, submitted).
The purpose of this study was to investigate the effects of a 4-month BWSTT programme on arterial dimension and function and measures of HRV and BPV in individuals with motor-complete SCI. In addition, as the physiological characteristics of our participants varied widely, we made a preliminary examination of what factors may predict favourable outcomes following BWSTT in individuals with motor-complete SCI.
Methods
Participants
Six individuals (four male, two female; age 37.7±15.4 years) with chronic SCI (C4-T12; ASIA A–B; 7.6±9.4 years post-injury) were included in the present investigation. Although 10 individuals were initially recruited, three participants withdrew from the study for personal reasons, and one participant could not adequately adhere to the training programme due to recurrent health issues. All participants were recruited from an outpatient SCI clinic at Chedoke Hospital, Hamilton, Ontario, Canada. Participants were only included if they were at least 1 year post-injury and were free of any coincident cardiac disease. Recent work has shown that the typical cardiovascular adaptations associated with SCI (decreased vessel diameters and blood flow) occur within the first 6 weeks postinjury, and therefore, our participants had likely reached a plateau in terms of these cardiovascular variables.35 Participants were also required to be free of any musculoskeletal condition that would contraindicate exercise training. This investigation was approved by the McMaster Medical Research Ethics Board (MREB), and participants provided written informed consent in accordance to MREB guidelines. We certify that all applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed during the course of this research. Participant characteristics are summarized in Table 1.
Testing protocol
Participants underwent two separate testing procedures at baseline and after 4 months of BWSTT. Testing session #1 consisted of Doppler ultrasound imaging of the carotid, femoral and brachial arteries. Testing session #2 consisted of continuous heart rate (HR) and blood pressure (BP) data acquisition to determine measures of HRV and BPV. For both sessions data were acquired during supine rest. In order to minimize external confounding stimuli, testing sessions were conducted on separate days, and post-testing sessions were always conducted at least 24 h after the final training session to ensure a true resting state. In general, testing sessions occurred between 1000 and 1700, however, for any given participant the time of day at each testing session never differed by more than 2 h at pre- and post-testing. Participants were instructed to abstain from caffeine and cigarette smoking for at least 12 h prior to testing, and were 2 h postprandial. Antiembolic stockings and abdominal binders were not worn during the testing sessions, and participants were asked to empty their urine bags prior to data acquisition. Medications were not interrupted during the study, however, medications and dosages were identical at all testing sessions for each participant. In addition, the only medications that may reasonably have affected our measures were baclofen (taken by five participants), and ditropan (taken by two participants), which were taken for their antispastic properties. The incidence of cardiovascular side effects associated with these medications are very low, and often transient and only associated with the start of treatment.36
Doppler ultrasound imaging
Upon entering the laboratory, participants were transferred onto a padded table and approximately 5–10 min of supine rest preceded any data acquisition. The common carotid, common femoral and brachial arteries were then imaged during supine rest.
Arterial diameters and mean blood velocities (MBV) were estimated by a Doppler ultrasound system with a high-resolution (5–10 MHz) linear array probe (GE Vingmed System Five), and BP was simultaneously recorded by a Finapress cuff for subsequent determination of arterial compliance (Ohmeda 2300, Madison, WI, USA). BP and MBV data were continuously recorded at 200 Hz and stored on a computer for later analysis with the use of customized software (Chart 4, Powerlab), and digital Doppler ultrasound images were stored on the system's internal hard drive for subsequent manual analysis (Echopac image analysis V=GE). The pulsed wave Doppler sample volume gate was adjusted to cover the width of the entire vessel to account for the nonuniform blood velocity distribution within the vessel. Real-time images were over two cardiac cycles, and therefore, systolic and diastolic diameters could be measured and used to determine mean vessel diameter ((systolic+2 (diastolic))/3). For each image obtained, systolic diameter was measured three times and averaged for a final value, and likewise for diastolic diameter. In addition, three images were taken for each artery and averaged. MBV was obtained by integration of the area under the curve of the continuous MBV signal over a 1-min period that included the time of the vessel imaging. The blood flow velocity of our system was calibrated against a corn-starch solution pumped through tubing.37
Arterial blood flow volume (BF) was calculated as: BF (ml/min)=MBV (cm/s) × mean vessel cross-sectional area (CSA; cm2) × 60; where CSA=π(mean diameter/2)2. Arterial resistance (mmHg min/ml) was calculated as: Resistance (mmHg min/ml)=mean arterial pressure (MAP)/BF. Arterial compliance was calculated as: Compliance (mm2/mmHg)=(systolic CSA (mm2) − diastolic CSA (mm2))/pulse pressure (mmHg).
HR and blood pressure data acquisition for HRV and BPV measures
Continuous recordings of HR and BP data were obtained from each participant at baseline and after 4 months of BWSTT. Upon entering the laboratory, each participant was transferred onto a padded table and fitted with a Polar HR monitor and a finger plethysmography (Finapres) cuff (Ohmeda 2300, Madison, WI, USA). In an attempt to achieve steady-state resting conditions participants lay quietly for 10 min prior to the start of data collection, which took place in a dark, quiet room during spontaneous breathing. HR and BP data were collected during a 20-min period of supine rest. Participants were asked not to sleep during data collection and although they were not disturbed during the testing sessions, none had any problems remaining awake. Systolic, diastolic and mean arterial pressures (MAP=(SBP+2DBP)/3) were determined by automated ausculation at the left brachial artery (Dinamap pro 100V2, GE Medical Systems, Tampa, FL, USA) just prior to the 20-min period of supine rest. All subsequent single-value BP data in the present study refer to these measures.
HR and Finapres BP signals were sampled at 500 Hz using a 12-bit analog-digital converter (CODAS, DATAQ Inc., Akron, OH, USA). The signals were continuously and simultaneously displayed on an IBM computer using WINDAQ data acquisition software (Dataq Instruments, Akron, OH, USA). The data were then saved on the computer hard drive and transferred to a separate computer (Daewoo AMD-K6 processor) equipped for HRV and BPV analysis.
Computation of HRV and BPV
A customized software program (MATLAB),38 was used to identify a stable and noise-independent fiducial point on all R-waves for each recording, as well as beat-to beat values of systolic blood pressure (SBP). An RR-interval tachogram, as well as a separate SBP tachogram, were then generated from the continuous HR and BP data, respectively. All tachograms were inspected for ectopic beats which were subsequently removed using a linear interpolation algorithm.38 When files were found to contain excessive ectopic beats (>5/min), the investigator visually inspected the tachogram for a sufficiently long period of relatively ectopic-free segments of HR and BP data for further analysis. Beat-to-beat HRV and BPV signals were then computed, and then resampled at 2 Hz using linear interpolation to obtain equally sampled time series. For each data set, eight record lengths of 256 points were selected automatically for power spectral analysis. The mean value of the HR and BP were removed and the equally sampled HRV and BPV signals were fed through a second-order high-pass Butterworth filter with a cutoff of 0.02 Hz. Power spectra were then computed from the filtered HRV and BPV signals using previously described software.38 The computations required to determine the power spectra are beyond the scope of this paper, however, the algorithms are provided in detail elsewhere.39
Final frequency domain measures represent the average of all accepted record lengths. Oscillations ranging between 0.04 and 0.15 Hz were designated as LF while oscillations between 0.15 and 0.40 Hz were designated as HF. The data analysis software used allowed the investigator to accept or reject any of the eight power spectra produced for each 20-min testing session. Thus, the investigator could reject power spectra showing a fusion of the LF and HF peaks, as sometimes, albeit rarely, occurred during spontaneous breathing. Peak values of the LF and HF components were identified from the HRV and BPV power spectra and expressed as (beats/min)2/Hz, and (mmHg)2/Hz, respectively. The power of the LF and HF components were calculated via integrating the area under each curve and expressed as (beats/min)2 and mmHg2.
Training intervention
BWSTT apparatus
The Woodway Loco-system (Woodway USA Inc., Foster, CT, USA) is a specialized treadmill with a built-in weight supporting system. Participants were fitted with a harness while seated in their wheelchairs and then wheeled up a ramp to the treadmill. Cables were then attached to the harness, and a pulley system was used to hoist participants into the standing position over the treadmill. Once upright, a second set of cables were used to connect participants to weight stacks located at the front of the treadmill which could be set at a predetermined percentage of each participant's body weight. The Woodway Loco-system allows for a range of speeds between 0.1 and 5.0 km/h, and speed may be adjusted by 0.1 km/h increments. Hand-rails are attached to the treadmill, and while participants could use these rails for balance if needed, they were discouraged to use them to assist in weight support. Two trainers sat at either side of the tread, and assisted participants in the gait cycle, while a third stood behind the participant and aided in weight shifting, balance and general safety.
Training protocol
Participants exercised at a frequency of three times per week for 4 months. During the first training session an appropriate amount of body-weight support was chosen for each participant, such that he or she could just stand on the tread without buckling at the knees. Although participants had the opportunity to decrease body-weight support as individually tolerated, none of them were able to reduce their support over the course of the training protocol. For the sake of safety, initial treadmill speed was arbitrarily chosen at 0.5 km/h, and the duration of ambulation at 15 min (three bouts of 5 min). As the exercise was essentially passive, speed and duration were progressed relatively quickly in the training programme. Increases in speed were largely dictated by the amount of spasticity in the legs, and the trainers' ability to move the leg through this spasticity while maintaining proper gait mechanics and safety. Time constraints dictated a maximum duration of 60 min. Daily training HR's were recorded in each participant's training log, from which an average training HR could be determined over the course of the 4-month training period. Also, in order to determine the contribution of upright posture to the HR response to ambulation, HR was determined after 5 min of motionless upright suspension on the treadmill at baseline and after the 4 months of BWSTT. The baseline upright HR was determined on the third day of training to allow for habituation to the BWS treadmill.
Statistical analysis
Potential exercise-induced changes in carotid and femoral artery dimension and function were determined via two-factor (vessel × time) analyses of variance (ANOVA) with the brachial artery serving a nonexercise control vessel. One-way ANOVA with repeated measures for time were used to determine potential exercise-induced changes in measures of HRV and BPV, and to compare the HR response to BWSTT between the first 2 months of training and the last 2 months. Tukey HSD post hoc analyses were used as required to determine specific differences between means and Pearson's r correlation analyses were performed to determine possible relationships between variables. Owing to the very preliminary nature of this research the level of statistical significance was set at P<0.10, and throughout the text and figures, data are presented as means±SD. In order to identify factors that may predict positive outcome, participants were divided into two groups based on the HR response to ambulation; that is, those who experienced an average training HR greater than 100 beats/min (responders), and those who experienced an average training HR less than 100 beats/min (nonresponders).
Results
Programme compliance and safety considerations
The compliance rate (number of sessions completed/number of scheduled sessions × 100) was 83.3±7.6%. One individual experienced frequent syncope during the training sessions, especially nearing the end of the 4-month protocol. One other participant developed a stage 1 pressure sore over a vertebrae due to irritation from the harness. The sore was detected early, covered with a clear adhesive during the subsequent training sessions and was not problematic. No episodes of autonomic dysreflexia or musculoskeletal injuries occurred in any participant during the course of the training programme.
Cardiovascular response to BWSTT
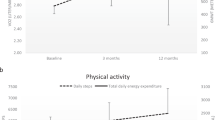
There was no significant change in resting HR following the 4-month BWSTT programme (Table 2), and therefore, pre- and post-values were averaged for the determination of the HR response to BWSTT. Likewise, there was no significant change in the average HR during BWSTT when comparing the first 2 months of training to the last 2 months, or upright HR at pre- and post-testing. Upright HR's were significantly higher than resting HR's (P=0.001), and there was a further modest but significant (P=0.09) increase in HR from the upright position to ambulation (resting: 64.4±6.3; upright: 80.0±11.6; ambulation: 98.1±22.3 beats/min). However, the HR response to ambulation was extremely varied in our participants, especially in those with tetraplegia. For example, participants with paraplegia experienced an average HR increase of 37.1±10.7% (range 28–49%; exercise HR: 86–106 beats/min) while those with tetraplegia experienced an average increase of 67.1±39.1% (range 26–104%; exercise HR: 68–135 beats/min). Peak exercise HR's recorded over the course of training (range 78–168 beats/min) were significantly higher than the average exercise HR (P=0.004). Three participants experienced an average exercise HR greater than 100 beats/min (responders: #2: 135, #4: 106, #6: 103 beats/min), the other three experienced a much more modest HR response to ambulation (nonresponders: #1: 68, #3: 86, #5: 93 beats/min). The distinction between responders and nonresponders should not be confused with the distinction between those with tetraplegia and those with paraplegia (see Table 1), that is, the HR response to ambulation was not simply a factor of lesion level. Rather, greater HR responses to ambulation seemed to occur in those with the greatest degree of muscle spasticity and in those who were the least tolerant of orthostatic stress. In particular, there was a significant correlation between the percentage increase in HR from rest to ambulation and the percentage decrease in MAP from rest to upright posture at baseline testing (P=0.02, r=0.89; Figure 1).
The effects of BWSTT on resting measures of arterial dimension and function
Two-way ANOVA revealed no significant vessel × time interactions suggesting no exercise-induced change in femoral or carotid artery cross-sectional area (Figure 2a; P=0.73 and 0.59, respectively), blood flow (Figure 2b; P=0.32 and 0.68, respectively) or resistance (Figure 2c; P=0.38 and 0.82, respectively) compared to the nonexercising brachial artery following the 4 months of BWSTT. In contrast, although there was no significant interaction suggestive of a change in carotid artery compliance (P=0.82), there was a significant vessel × time interaction (P=0.07) indicating an exercise-induced increase in femoral artery compliance (Figure 2d). Finally, there were no apparent differences between responders and nonresponders for exercise-induced changes in arterial dimension or function.
The effects of BWSTT on resting measures of HRV
There were no significant changes in resting LF power, HF power or the LF:HF ratio after 4 months of BWSTT (P=0.11, 0.47 and 0.25, respectively) (Table 2). There was, however, a suggestion that responders may have experienced a selective, but not statistically significant, training-induced decrease in the LF:HF ratio (pre: 1.53±0.67 versus post: 1.04±0.35; P=0.14), while nonresponders showed no change (pre: 1.37±0.08 versus post: 1.41±0.35; P=0.85) (Figure 3). The putative reduction in the LF:HF ratio among responders was due to increases in HF power (4272.0±785.3 versus 5203.4±845.6 (beats/min)2; P=0.03) and nonsignificant decreases in LF power (5845.7±1727.0 versus 4975.8±868.4 (beats/min)2; P=0.27).
The effects of BWSTT on resting measures of BPV
There was no change in resting SBP, DBP or MAP following the 4 months of BWSTT (P=0.90, 0.62 and 0.74, respectively) (Table 3). Likewise, there were no significant training-induced changes in LFSBP (P=0.64) (Table 3). However, responders seemed to experience selective and significant training-induced decreases in LFSBP (responders pre: 196.3±20.0 mmHg2, responders post: 158.5±13.7 mmHg2, P=0.03; nonresponders pre: 131.7±48.7 mmHg2, nonresponders post: 153.5±79.2 mmHg2, P=0.36) (Figure 4).
Discussion
The main finding of this study is that individuals with motor-complete SCI may experience positive peripheral vascular changes in the exercising lower limbs following 4 months of BWSTT as evidenced by the increase in femoral artery compliance following the training programme. This change also appeared to be quite robust as it was observed in five of our six participants with one individual experiencing no change. The borderline statistical significance in the femoral compliance measure was likely due to our small sample size, as the effect size of this adaptation was very large (ES=1.01). The HRV and BPV data were not quite as clear and suggested that only a select group of individuals may experience changes in these measures following BWSTT.
The effects of BWSTT on vascular dimension and function
The factors contributing to the suggested increase in femoral artery compliance appeared to be independent of the HR response to exercise, as responders and nonresponders showed similar changes. Thus, BWSTT may promote changes in peripheral vascular compliance in motor-complete SCI despite the relatively low HR that may be elicited. The lack of a change in carotid artery compliance suggests, however, that although BWSTT was effective in causing selected local vascular effects it was not an intense enough exercise stimulus to cause central vascular adaptations.
From a clinical perspective, the observed increase in femoral artery compliance may be encouraging for individuals with complete SCI, as the relationship between arterial stiffness and cardiovascular risk has been well established.23, 40, 41 Specifically, the inability of a vessel to expand and recoil with pressure changes results in damage to the vessel wall, which in turn may lead to atherosclerosis and thrombus formation.23 Vessel compliance also converts intermittent blood flow from the heart to a more steady flow throughout the circulation and thus, enables a more effective tissue perfusion. As individuals with complete SCI are at an increased risk of complications due to thrombus formation, and pressure sore development due to poor tissue perfusion,1 exercise-induced increases in arterial compliance may be particularly desirable in this population. Thus, although BWSTT may not enhance ambulation in individuals with complete SCI, it may promote clinically relevant improvements in vascular compliance and should be encouraged as a possible rehabilitation technique in this population.
The true value of BWSTT as a means of promoting positive vascular adaptation must be made in comparison to other currently available modalities for individuals with complete SCI. First, although arm ergometry may promote central cardiovascular changes in those with sufficient arm function, this form of exercise becomes less beneficial in individuals with cervical lesions.8 Further, as leg blood flow actually decreases during arm ergometry, even in individuals with SCI (albeit to a lesser extent),42 vascular changes of the lower limbs would not be expected with this form of exercise. In contrast, FES exercise has proven to be an effective means of improving peripheral cardiovascular function in individuals with SCI. For example, Hopman et al6 found an approximate 30% decrease in femoral artery resistance following 6 weeks of FES cycling and an approximate 30% increase in femoral artery blood flow in nine participants with complete SCI. Similarly, in a study conducted by Gerrits et al5 6 weeks of FES cycling was associated with significant increases in femoral artery diameter (8%) and resting mean inflow volume (37%), and a significant decrease in femoral artery resistance (8%). No changes were noted, however, in the carotid artery. Finally, Nash et al7 examined the effects of electrically stimulated ambulation exercise and found a significant 33% increase in femoral artery cross-sectional area, accompanied by a significant 56% increase in resting femoral artery blood flow. While, measures of arterial compliance were not included, the combination of stimulated contractions and upright posture may prove to be the most effective means of promoting cardiovascular adaptation in individuals who can tolerate this modality. Still, FES exercise carries the risk of skin irritation, autonomic dysreflexia and bone fracture, and as mentioned, stimulated contractions may not be evoked in those with lower motor neuron injuries.8 Thus, BWSTT may be an effective alternative for promoting positive vascular adaptations in individuals with SCI who cannot tolerate or do not have access to FES exercise. It is of interest to note that the present study did include one participant with a lower motor neuron injury (#3), and this individual experienced substantial increases in femoral artery compliance and blood flow. Anecdotally, this participant also reported an improved temperature regulation of the legs and an improved wound healing ability. Although, these are only single case observations they are highly encouraging as BWSTT may prove to be a unique means of improving vascular function in individuals with lower motor neuron injuries. Further research is certainly warranted to investigate this possibility.
The effects of BWSTT on HRV and BPV
In general, BWSTT did not prove to be a major central cardiovascular stimulus for individuals with motor-complete SCI, and as such, our participants as a whole did not experience significant changes in measures of HRV and BPV. As mentioned, peak exercise HR's recorded over the course of training were significantly higher than the average exercise HR's (P=0.004), and in fact only one participant failed to experience a peak HR greater than 110 beats/min. This suggests that the passive nature of the movement was more likely responsible for the relatively modest HR response to ambulation, rather than sympathetic decentralization per se which would preclude exercise HR's in excess of approximately 110 beats/min. However, despite the small sample size, the results suggest that a subgroup of individuals may experience a substantial HR response to this form of exercise, despite its involuntary nature, and accordingly may experience modest changes in measures of HRV and BPV. Thus, individuals with motor-complete SCI who respond to BWSTT may experience relative increases in the vagal outflow to the heart and decreases in BPV that have been associated with decreased risk of cardiovascular mortality and end-organ damage, respectively.32, 33, 43 In the present study, the factors accounting for the HR response to BWSTT seemed to be the presence of muscular spasticity and a propensity to orthostatic intolerance. The relationship between postural hypotension and the HR response to BWSTT was clear and demonstrated by the significant correlation between the decrease in MAP upon upright posture and the increase in HR from rest to ambulation. This relationship suggests that the upright nature of BWSTT is a key component to its potential benefits, as it necessitates a HR response in those intolerant of postural stress. Further support for this contention may be that recumbent passive exercise has been shown to evoke no HR response in individuals with SCI.44 However, the upright posture did not account for the entire HR response to ambulation since HR's tended to be higher during BWSTT than during motionless upright posture. Factors accounting for this further increase in HR may only be speculated upon. Unfortunately, measures of muscle spasticity were not conducted during ambulation, and therefore, its contribution to the HR response is more anecdotal but certainly clear during the training sessions. Further research is required to examine this issue.
The autonomic adaptations that may be achieved via BWSTT should be compared to those that may be achieved by other available forms of exercise for individuals with SCI. Such comparisons are difficult due to the scarcity of literature in this area, however, previous work from our lab has shown a 38% reduction in the LF:HF ratio following 3 months of combined resistance and arm ergometry in individuals with incomplete tetraplegia (Ditor et al, unpublished observations); a comparable change to that observed by the responders in the present study. However, the participant characteristics varied greatly between these two studies and further research is required to determine the comparative efficacy of these two training techniques. In summary, peripheral vascular changes were associated with BWSTT regardless of the HR response to ambulation. Therefore, those who do not experience substantial increases in HR during ambulation may still achieve vascular benefit from BWSTT, but may wish to supplement this exercise with arm ergometry in order to achieve improvements in neurocardiac and neurovascular control.
Conclusions
The present study is the first to provide evidence that BWSTT is associated with increased femoral artery compliance in individuals with motor complete SCI. Further, this improvement in vascular function may be achieved regardless of the HR response during ambulation. BWSTT should therefore be encouraged as a means of improving cardiovascular health in this population, especially in those who cannot tolerate or do not have access to FES exercise. BWSTT may also cause modest improvements in measures of HRV and BPV in a select subgroup of individuals who respond to ambulation with moderate to large increases in HR. In the present study, factors associated with a substantial HR response to BWSTT seemed to be a propensity to orthostatic intolerance and muscular spasticity.
References
DeVivo MJ, Black KJ, Stover SL . Causes of death during the first 12 years after spinal cord injury. Arch Phys Med Rehabil 1993; 74: 248–254.
Groah SL, Weitzenkamp D, Sett P, Soni B, Savic G . The relationship between neurological level of injury and symptomatic cardiovascular disease risk in the aging spinal injured. Spinal Cord 2001; 39: 310–317.
Mammen EF . Pathogenesis of venous thrombosis. Chest 1992; 102(Suppl 6): 640S–644S.
Nash MS, Montalvo BM, Applegate B . Lower extremity blood flow and responses to occlusion ischemia differ in exercise trained and sedentary tetraplegic persons. Arch Phys Med Rehabil 1996; 77: 1260–1265.
Gerrits HL, de Haan A, Sargeant AJ, van Langen H, Hopman MT . Peripheral vascular changes after electrically stimulated cycle training in people with spinal cord injury. Arch Phys Med Rehabil 2001; 82: 832–839.
Hopman MTE, Groothuis JT, Flendrie M, Gerrits KHL, Houtman S . Increased vascular resistance in paralyzed legs after spinal cord injury is reversible by training. J Appl Physiol 2002; 93: 1966–1972.
Nash MS, Jacobs PJ, Montalvo BM, Klose JK, Guest RS, Needham-Shropshire BM . Evaluation of a training program for persons with SCI paraplegia using the parastep 1 ambulation system: Part 5. Lower extremity blood flow and hyperemic responses to occlusion are augmented by ambulation training. Arch Phys Med Rehabil 1997; 78: 808–814.
Jacobs PL, Nash MS . Modes, benefits, and risks of voluntary and electrically induced exercise in persons with spinal cord injury. J Spinal Cord Med 2001; 24: 10–18.
Castro MJ, Apple DF, Staron RS, Campos GE, Dudley GA . Influence of complete spinal cord injury on skeletal muscle within 6 months of injury. J Appl Physiol 1999; 86: 350–358.
Ditor DS et al. Na+,K+-ATPase concentration and fibre type distribution after spinal cord injury. Muscle Nerve 2004; 29: 38–45.
Olive JL, Slade JM, Dudley GA, McCully KK . Blood flow and muscle fatigue in SCI individuals during electrical stimulation. J Appl Physiol 2003; 94: 701–708.
Barbeau H, Danakas M, Arsenault B . The effects of locomotor training in spinal cord injured subjects: a preliminary study. Restorative Neurol Neurosci 1993; 5: 81–84.
Protas EJ, Holmes A, Qureshy H, Johnson A, Lee D, Sherwood AM . Supported treadmill ambulation training after spinal cord injury: pilot study. Arch Phys Med Rehabil 2001; 82: 825–831.
Wernig A, Muller S . Laufband locomotion with body weight support improved walking in persons with severe spinal cord injuries. Paraplegia 1992; 30: 229–238.
Wernig A, Muller S, Nanassy A, Cagol E . Laufband therapy based on ‘rules of spinal locomotion’ is effective in spinal cord injured persons. Eur J Neurosci 1995; 7: 823–829.
Wernig A, Nanassy A, Muller S . Laufband (treadmill) therapy in incomplete paraplegia and tetraplegia. J Neurotrauma 1999; 16: 719–726.
Beherman AL, Harkema SJ . Locomotor training after human spinal cord injury: a series of case studies. Phys Ther 2000; 80: 688–700.
Dietz V, Colombo G, Jensen L, Baumgartner L . Locomotor capacity of spinal cord in paraplegic patients. Ann Neurol 1995; 37: 574–582.
Dietz V, Wirz M, Jensen L . Locomotion in patients with spinal cord injuries. Phys Ther 1997; 77: 508–516.
Schmidt-Truckass A et al. Arterial properties of the carotid and femoral artery in endurance-trained and paraplegic subjects. J Appl Physiol 2000; 89: 1956–1963.
Walden R, Bass A, Ohry A, Schneiderman J, Adar R . Pulse volume recording disturbances in paraplegic patients. Paraplegia 1991; 29: 457–462.
Arnett DK, Evans GW, Riley WA . Arterial stiffness: a new cardiovascular risk factor? Am J Epidemiol 1994; 140: 669–682.
Seals DR . Habitual exercise and the age-associated decline in large artery compliance. Exerc Sport Sci Rev 2003; 31: 68–72.
Akselrod SD, Gordon FA, Ubel DC, Shannon A, Berger C, Cohen RJ . Power spectrum analysis of heart rate fluctuation: a quantitative probe of beat-to-beat cardiovascular control. Science 1981; 213: 220–222.
Kamath MV, Fallen EL . Power spectral analysis of heart rate variability. A non-invasive signature of cardiac autonomic function. CRC Crit Rev Biomed Eng 1993; 21: 245–311.
Pagani M et al. Relationship between spectral componenets of cardiovascular variabilities and direct measures of muscle sympathetic nerve activity in humans. Circulation 1997; 95: 1441–1448.
Parati G, Saul JP, Di Rienzo M, Mancia G . Spectral analysis of blood pressure and heart rate variability in evaluating cardiovascular regulation. Hypertension 1995; 25: 1276–1286.
Pomeranz B et al. Assessment of autonomic function in humans by heart rate spectral analysis. Am J Physiol 1985; 248: H151–H153.
Montano N, Ruscone TG, Porta A, Lombardi F, Pagani M, Malliani A . Power spectrum analysis of heart rate variability to assess the changes in sympathovagal balance during graded orthostatic tilt. Circulation 1994; 90: 1826–1831.
Ponikowski P et al. Depressed heart rate variability as an independent predictor of death in chronic congestive heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol 1997; 79: 1645–1650.
Kleiger RE, Miller JP, Bigger JT, Moss AJ, the Multicentre post-infarction research group. Decreased heart rate variability and its association with increased mortality after acute myocardial function. Am J Cardiol 1987; 59: 256–262.
Frattola A, Parati G, Cuspidi C, Albini F, Mancia G . Prognostic value of 24 h blood pressure variability. J Hypertens 1993; 11: 1133–1137.
Miao C, Su D . The importance of blood pressure variability in rat aortic and left ventricular hypertrophy produced by sinoaortic denervation. J Hypertens 2002; 20: 1865–1872.
Ditor DS, Kamath M, MacDonald MJ, Bugaresti J, McCartney N, Hicks AL . The effects of body-weight supported treadmill training on heart rate variability and blood pressure variability in individuals with spinal cord injury. J Appl Physiol 2005; 98: 1519–1525.
de Groot PC, Van Kuppevelt DH, Pons C, Snoek G, Van Der Woude LH, Hopman MT . Time course of arterial vascular adaptations to inactivity and paralyses in humans. Med Sci Sports Exerc 2003; 35: 1977–1985.
Canadian Pharmaceutical Association. Drugs. In: Hughes FH (ed). Compendium of Pharmaceuticals and Specialties. Canadian Pharmaceutical Association: Toronto, Ontario 2003.
Shoemaker JK, Pozeg ZI, Hughson RL . Forearm blood flow by Doppler ultrasound during rest and exercise: tests of day-to-day repeatability. Med Sci Sports Exerc 1996; 28: 1144–1149.
Kamath MV, Hollerbach S, Bajwa A, Fallen EL, Upton ARM, Tougas G . Neurocardiac and cerebral responses evoked by esophageal vago-afferent stimulation in humans: effect of varying intensities. Cardiovasc Res 1998; 40: 591–599.
Kay SM, Marple SL . Spectrum analysis. A modern perspective. Proc IEEE 1981; 69: 1380–1419.
Kingwell BA . Large artery stiffness: implications for exercise capacity and cardiovascular risk. Clin Exp Pharmacol Physiol 2002; 29: 214–217.
Safar ME . Systolic blood pressure, pulse pressure and arterial stiffness as cardiovascular risk factors. Curr Opin Nephrol Hypertens 2001; 10: 257–261.
Hopman MTE . Circulatory responses during arm exercise in individuals with paraplegia. Int J Sports Med 1994; 15: 126–131.
Bosner MS, Kleiger RE . Heart rate variability and risk stratification after myocardial infarction. In: Malik M, Camm AJ (eds). Heart Rate Variability. Futura Press: New York, NY 1995.
Figoni SF et al. Physiologic responses of paraplegics and quadriplegics to passive and active leg cycle ergometry. J Am Paraplegia Soc 1990; 13: 33–39.
Acknowledgements
We gratefully acknowledge the training assistance provided by the many undergraduate and graduate Kinesiology students. DS Ditor was the recipient of a studentship from the Ontario Neurotrauma Foundation. Dr MV Kamath also thank the DeGroote Foundation and NSERC, Canada.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ditor, D., MacDonald, M., Kamath, M. et al. The effects of body-weight supported treadmill training on cardiovascular regulation in individuals with motor-complete SCI. Spinal Cord 43, 664–673 (2005). https://doi.org/10.1038/sj.sc.3101785
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.sc.3101785
Keywords
This article is cited by
-
Functional Electrical Stimulation: Cardiorespiratory Adaptations and Applications for Training in Paraplegia
Sports Medicine (2015)
-
Autonomic Nervous System Dysfunction Following Spinal Cord Injury: Cardiovascular, Cerebrovascular, and Thermoregulatory Effects
Current Physical Medicine and Rehabilitation Reports (2015)
-
H-FABP, cardiovascular risk factors, and functional status in asymptomatic spinal cord injury patients
Herz (2013)
-
A systematic review of exercise as a therapeutic intervention to improve arterial function in persons living with spinal cord injury
Spinal Cord (2011)
-
Leg skin temperature with body-weight-supported treadmill and tilt-table standing training after spinal cord injury
Spinal Cord (2011)