Abstract
Study design:
Prospective, randomised controlled trial.
Objective:
To evaluate the effect of alendronate on bone mineral density in chronic spinal cord injury (SCI) patients.
Setting:
University-based rehabilitation centre in São Paulo, Brazil.
Methods:
A total of 19 chronic SCI patients were evaluated, divided into a control group and an experimental group. Control group patients received 1000 mg of calcium daily, and experimental group patients received 1000 mg of calcium plus 10 mg of alendronate daily. The study duration was 6 months. In all, 12 densitometric parameters were analysed using whole-body dual-energy X-ray absorptiometry at baseline and after 6 months.
Results:
The experimental group presented increases in nine densitometric parameters, although statistical significance was attained in only two of those parameters. In the control group, an increase was observed in only one parameter, whereas the remaining 11 presented either no alteration or a decrease.
Conclusion:
The use of alendronate had a positive effect on bone mineral density in SCI patients and therefore represents a potential tool for prevention and treatment of osteoporosis in this population.
Similar content being viewed by others
Introduction
Osteoporosis is a well-known chronic complication of spinal cord injury (SCI).1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12 However, many challenges remain, such as gaining a better understanding of the physiopathology and optimising management. Bone loss mechanisms in SCI are not completely understood.1, 3, 4 In SCI, a significant portion of bone loss occurs during the first 4–6 months after injury (30% within the first 3–4 months) and stabilises between the 12th and 16th months.1, 2, 5, 6 Bone loss initially affects the whole body and is later restricted to paralysed areas.7, 8, 9, 10
Lack of mechanical stress is certainly involved in, but cannot be held solely responsible for, the intensity of bone loss. Neurovascular changes secondary to modification of the autonomic nervous system, as well as resistance to or decreased levels of insulin-like growth factor 1, have been implicated in this process.13 The fact that the bone loss seen in SCI is more intense than that resulting from immobility can be attributed to the complexity of the mechanisms involved.5 It has also been suggested that SCI may cause structural changes in collagen, inducing increased resorption.6, 13 Immobilisation-related bone loss was first described in 1941 by Albright et al14 as an atrophy of the bone, simulating hyperparathyroidism. As a result of the bone loss, there is decreased bone density and a consequent increase in fracture risk. The incidence of pathologic long-bone fractures in SCI has been reported to be between 1 and 7%.15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25 Such fractures typically result from minor traumas occurring during daily activities,16 stretching exercises,17 transfers,18 or the use of functional electrical stimulation (FES).19 In the majority of cases, the patient has no recollection of the trauma mechanism.20
Regarding prevention and treatment of osteoporosis in SCI patients, no effective management strategy has been established to date. Studies endeavouring to evaluate the role of orthostatism and exercise as a means of treatment and prevention have produced inconclusive results.26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37 Most studies using FES have shown little or no change in bone density.31, 33, 34, 36 The results of some studies have suggested local benefits.32, 35, 37
As far as medical treatment is concerned, the use of bisphosphonates constitutes a safe and effective therapeutic intervention for the treatment of osteoporosis,38, 39, 40 although there have been very few studies involving SCI patients.41, 42, 43, 44 The mechanisms of action of bisphosphonates such as alendronate, clodronate, etidronate, pamidronate, risedronate, and tiludronate include reduction of bone resorption through osteoclast inhibition and long-term reduction of the number of osteoclasts. The bisphosphonate etidronate also interferes with bone mineralisation and is therefore used cyclically in order to avoid this undesirable effect.
Pearson et al41 evaluated the effect of cyclic etidronate (2-week regimens of 800 mg a day, with a 13-week interval intervening) for 30 weeks (two cycles) in six SCI patients compared with seven SCI controls and found benefit for those that recovered mobility. Minaire et al42 studied the effect of dichloromethylene in 21 paraplegic patients and concluded that it can reduce bone loss during the acute phase. Another study included 70 patients with immobilisation-related bone loss and compared different treatments: calcitonin (n=20), etidronate (n=20), placebo (n=16), and clodronate 400 mg/day (n=7) or 1600 mg/day (n=7).43 The authors found that calcitonin and the 1600 mg/day dose of clodronate appeared to be the most effective for inhibiting bone resorption. Chappard et al44 studied the effect of 200 and 400 mg/day of tiludronate in 20 paraplegic patients and found that, compared to controls, patients benefited from 400 mg/day of tiludronate.
In their favour, bisphosphonates also act specifically on the bone tissue and have minimal side effects, which can include gastrointestinal disturbances and (quite rarely) hypersensitivity reactions.38
Prevention and treatment of osteoporosis through pharmacological means shows promise. Such treatment should, ideally, be introduced early since orthostatism and exercise alone have not been proven effective. Szollar21 suggested that preventive intervention should be initiated approximately 1 year after injury and, should the densitometric evaluation show significant bone loss before that period, treatment should be started even earlier. The author also suggested semestral densitometric evaluation.
Bearing in mind the above findings, together with those of the few studies evaluating the use of bisphosphonates in managing osteoporosis in this group of patients, this study was undertaken with the objective of evaluating the effect of alendronate on bone mineral density (BMD) in chronic SCI patients. The aim was to assess alendronate as a possible therapeutic intervention for minimising the accentuated bone loss that occurs in these patients and the complications arising from the same. It should be emphasised that alendronate has been proven to be safe and effective in the treatment of osteoporosis in both men and women and has been approved by the Food and Drug Administration.38
Methods
Study design and procedure
A controlled randomised clinical study was conducted in order to compare SCI patients treated with alendronate and calcium (experimental group) with SCI patients treated with calcium alone (control group). The patients were recruited from those treated at the Spinal Cord Outpatient Clinic of the Department of Rehabilitation Medicine of the University of São Paulo during the period from May to September of the year 2000.
In order to eliminate any influence of voluntary mobility, the study included only patients with SCI for more than 6 months and presenting class A, B, or C injuries as designated by the American Spinal Injury Association (ASIA) classification system.45 Both female and male patients were included, although age cutoffs were imposed (35 for females and 50 for males) in order to avoid the physiological bone loss period. Subjects were excluded if presenting any of the following conditions: active heterotopic ossification; renal, metabolic or liver disease; alcoholism; pregnancy; breast feeding; chronic use of steroids, heparin or anticonvulsants; smoking; or recent radiological exposure. Patients were submitted to initial clinical evaluation (interview and physical examination) and laboratory tests in order to rule out secondary causes of osteoporosis.
Control group patients received 1000 mg of calcium daily, divided in two doses. Patients in the experimental group also received 1000 mg of calcium daily, divided in two doses, plus a daily dose of 10 mg of alendronate. Patients in both groups continued these regimens, without interruption, for 6 months. The patients who received the alendronate were instructed to take it with water on an empty stomach, not to lie down for at least 30 min after having taken the medication and not to take any other medicine, including the calcium, simultaneously. Patients were submitted to densitometric evaluation at the study onset and again at the study end point (after 6 months). In addition, all patients were examined, on a monthly basis, throughout the study period. Whole-body bone density was measured through dual-energy X-ray absorptiometry (DEXA) using a Lunar Model DPX (Lunar Corp., Madison, WI, USA). The Ethics Committee of the University of São Paulo Hospital das Clínicas approved the experimental protocol, and all participating patients gave written informed consent.
Measurements and analysis of data
Bone loss was assessed through evaluation of the following three bone density parameters: BMD (expressed as g/cm2); T index (variation in relation to young adults; expressed as mean and standard deviation, SD); Z index (variation in relation to individuals in the same age bracket; expressed as mean and SD). In addition to whole body measurements, upper-extremity (UE), lower-extremity (LE), and trunk measurements were also taken.
Subsequent to the initial measurements, the means were compared using Student's t-test with the purpose of assuring that both groups of patients would be comparable in terms of the features of interest to the researcher and determining whether the randomisation had been successful. Variations between initial and final bone density measurements were then calculated, as was the mean of the difference in variation of all bone density parameters. The means of variation of both groups were compared using Student's t-test (null hypothesis: the means of variation of both groups would be equal). The level of statistical significance adopted was P<0.05. The statistical analysis was carried out using the statistical data analysis software program STATA, version 7.0 (Stata Corp., College Station, TX, USA).46
Results
A total of 19 patients were included in the study. In all, 10 patients were randomised to receive alendronate, and nine were randomised to the control group. Each group included two women, corresponding to 22.2% of the control group and 20% of the experimental group (P=0.91). The two groups were also similar in age, weight, and time since injury (Table 1).
Regarding the degree and nature of the injury, the control group consisted of four paraplegics and five quadriplegics, among whom there were four victims of automobile accidents, three gunshot victims, and two victims of falls. The treatment group consisted of eight paraplegics and two quadriplegics, among whom there were five victims of gunshots, two victims of automobile accidents, three victims of falls, and one victim of an infectious disease. All patients tolerated 10 mg of alendronate without any reported adverse effects. One patient in the control group presented constipation that was promptly managed with conservative treatment.
Two patients (one in each group) interrupted their participation in the study and were not submitted to the final densitometric evaluation and were thus excluded from the final data analysis. One of those two patients moved to another city during the treatment period, and the other failed to report for the monthly appointments from the 3rd month onwards because of a job change.
Mean initial BMD measurements were similar for all body segments in both groups. Mean T and Z indexes obtained in the original evaluation were also comparable. In both groups, initial LE indexes were the lowest and were lower in the experimental group patients than in the controls. In contrast, initial UE indexes were the highest in both groups.
After 6 months of treatment, patients in the experimental group presented increased bone density in nine of the 12 parameters analysed. In the three remaining parameters (LE BMD, total BMD, and LE T index), there was practically no variation. In the control group patients, only the LE Z index showed a positive variation, whereas there was either no change or a loss of bone density in the remaining parameters. The greatest difference in variation between the two groups was found in the UE T index, in which control group patients presented a mean loss of 0.63 and experimental group patients presented a mean gain of 0.29 (Table 2). The least difference in variation was found in the LE index.
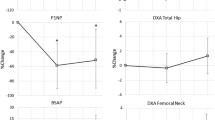
Comparison of variations in mean UE BMD showed that there was a consistent increase in bone density in patients treated with alendronate plus calcium (0.03), whereas control group patients presented greater variability in UE BMD values, which were higher in some and lower in others (mean, −0.03). Mean increase in UE BMD in the experimental group was greater than in the control group in a marginally significant manner (P=0.14; Table 2). No significant difference between the two groups was found in the BMD variation at the trunk (P=0.54). In both groups, there was wide variability, although patients in the experimental group presented an overall gain in bone density. Comparison of variations in LE BMD showed very similar values in both groups (P=0.73), practically unaltered after 6 months. In experimental group patients, there was a small increase in mean total BMD (0.01) that was slightly higher than that seen in control group patients (P=0.04; Table 2). Variations in the T index were quite similar to those found in the BMD analysis. In the experimental group, there was a greater increase in the mean UE T index (0.29) than in the control group (P=0.10). There was a tendency towards increased trunk T indices in experimental group patients (mean, 0.14), whereas trunk T indices presented a downward trend in control group patients (mean, −0.16), although the difference was not statistically significant (P=0.18; Figure 1). Comparison of variations in LE T indices revealed little change in either group (P=0.28). In the comparison of variations in total T indices, we observed an increase in the experimental group (mean, 0.14) and an overall decrease in the control group (mean, −0.16; P=0.04; Figure 2; Table 2).
In addition, the comparison of variations in the UE Z index showed a marginally significant difference between the two groups, increased in the experimental group (mean, 0.33) and decreased in the control group (mean, −0.44; P=0.09; Table 2). No statistically significant difference was found between the groups in trunk Z index variation, although patients in the experimental group also presented a mean increase in this index. Comparison of variations in the LE Z index revealed no significant differences between the groups (P=0.97). Patients treated with calcium plus alendronate presented greater increases in this index and less intragroup variability when compared to those treated with calcium alone. Finally, there was a marginally significant difference between the two groups in terms of total Z index variation, in which the experimental group presented an increase (mean, 0.21), whereas the control group presented a decrease (mean, −0.13; P=0.11; Table 2).
Discussion
Ideally, a study of bone metabolism should include measurement of biochemical reabsorption and determination of bone formation marker levels,47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58 which are more sensitive than bone densitometry, as has been shown by Machado et al.59 The authors compared the sensitivity of biochemical markers with that of bone densitometry in the evaluation of alendronate treatment in 26 postmenopausal women. They determined that the benefit for patients in the treated group, in comparison to controls, was statistically significant (P<0.01) in five of the six biochemical markers evaluated but in only two of the six regions evaluated by the densitometry. The authors also suggested that densitometric measurements should be taken 1 week before and after treatment in order to reduce short-term variability. However, these results corroborate the consensus in the literature that DEXA is a highly precise and reproducible method.60, 61, 62, 63, 64 In contrast, Liu et al,65 in a study of 64 chronic SCI patients, postulated that quantitative computed tomography is more sensitive than DEXA in evaluating spinal osteoporosis following SCI.
One of the main methodological limitations of the present study is the small number of subjects studied. Although patients in the experimental group performed better in the majority of bone density parameters, a satisfactory level of statistical significance was achieved in only two of those parameters. However, our findings suggest that treatment with alendronate can improve densitometric measurements. Another limitation is the fact that only initial and final bone density measurements were obtained. Therefore, we can only estimate the pattern of the effect of alendronate on bone mass. For example, the alterations in bone mass may occur in a constant form over time or may only begin some months after the initiation of treatment.
Ideally, the duration of treatment time should be longer, which would allow a more accurate evaluation. However, in the Fosamax International Trial,54 carried out in 153 centres in 34 countries and involving a total of 1908 patients with postmenopausal osteoporosis, sodium alendronate was compared to a placebo through analysis of biochemical markers and densitometric parameters. The authors found a considerable statistically significant difference between the two groups after 3 months of treatment, both in relation to biochemical markers and densitometric indices (lumbar spine and femur), and a progressive difference throughout the first year. Rossini et al66 obtained similar results when evaluating daily administration of 20 mg of alendronate for 6 months to 15 patients with postmenopausal osteoporosis, showing a 4.6% gain in bone mass at the lumbar spine level after 1 year. In addition, Adami et al67 compared daily administration of 10 mg (68 patients) and 20 mg (72 patients) of alendronate for 1 year and showed 4.4 and 5.8% gains in bone mass, respectively, at the lumbar spine level. In the present study, the best response to alendronate was achieved at the UE level, suggesting that treatment response is variable, and areas with functional mobility might present a greater response. Supplementary vitamin D, together with calcium, is indicated by some authors, but it is only advisable in elderly patients or patients with vitamin D deficiency.
Bearing in mind the evolution and particularities of the osteoporosis occuring in SCI patients, one should pay special attention to the time of injury when evaluating patients presenting similar rates of bone loss and comparing different therapeutic interventions. Intervention must, ideally, be introduced early, as a large portion of bone loss occurs within the first 16 months. During the first year, bone loss in paralysed areas is approximately 4% per month in areas rich in trabecular bone and 2% per month in those rich in cortical bone. In the upper portion of the tibia, an area particularly rich in trabecular bone, bone loss reaches 50% after 18 months, compared with 20% in the area close to the femur during the same period.9
Fracture incidence is considerable in SCI patients and may lead to autonomic dysreflexia, deformity promoting pressure ulcers, and additional loss of function. In such patients, fracture diagnosis is more challenging due to the sensory and functional deficits already present.16 Minor symptoms such as local sweating, oedema, and increased spasticity might be the only indications of fracture.18
Lazo et al15 found that risk of fracture is at least doubled for each unit decrease in T index BMD SD at the femoral neck, and concluded that measurement of BMD at this site can be used to quantify fracture risk in SCI patients. Szollar et al22 compared BMD measurements of 176 SCI patients with those of 60 patients with no injury. The authors studied the lumbar spine and femoral areas and found a significant decrease in bone density, reaching fracture thresholds after 1–5 years. However, in the lumbar spine, there was no significant decrease, either in quadriplegic or paraplegic patients. Dissociation of demineralization between the spine and pelvis is considered a typical finding in SCI patients,3, 4, 8 in contrast with endocrinopathies, which lead to greater bone loss at the spine. It has been suggested that relative preservation of bone density at the spine is due to the load provided by the sitting position.8
Regarding treatment, there have been few studies to date evaluating the use of kinesiotherapy. The few studies available typically involved small patient samples and short follow-up periods, which makes it difficult to evaluate possible benefits. Kunkel et al,26 in a study involving a group of SCI patients, found no benefit from 90 min per day of orthostatism with the use of an orthostatic board. Saltzstein et al28 evaluated subjects with complete and incomplete SCI in order to determine whether there was a correlation between mobility and bone density and stated that SCI individuals benefit from making efforts to stand with some regularity. Goemaere et al29 reported that subjects standing regularly, starting 1 year after SCI, had more well-preserved BMD than did age- and sex-matched controls. In addition, de Bruin et al30 suggested that an early exercise programme in which standing exercises are combined with walking seems to reduce the expected rate of bone loss in SCI patients, although the authors found no difference between combined early standing and walking and early standing only.
Studies involving FES have also yielded inconclusive results and very few have used acceptable methodology.31, 32, 33, 34, 35, 36, 37 Sipski et al32 evaluated the use of FES of the lower extremity muscles for 1 h five times a week plus half an hour three times a week on a REGYS® bicycle ergometer in two patients and observed some relative reduction of bone loss at the femur. Mysiw et al33 evaluated the use of 12 weeks of FES-induced bicycle ergometry in five patients and observed a reduction in hypercalciuria but not in bone loss intensity. Leeds et al34 evaluated the use of FES-induced bicycle ergometry for 6 months and found no significant benefit in terms of reduced bone loss. In contrast, Needham-Shropshire et al31 evaluated the effect of the use of the PARASTEP® system (an orthosis with FES) in paraplegic patients and observed no reduction in the intensity of bone loss. In addition, Mohr et al35 observed increased BMD in the proximal tibia but not in the femoral neck or lumbar spine, suggesting site-specific changes. Furthermore, BeDell et al36 studied the effects of FES-induced lower extremity cycling on the bone density of 12 chronic SCI patients and found no significant increase in bone density at the hip and some relative increase at the lumbar spine. Moreover, Bélanger et al,37 after studying 14 SCI subjects for 24 weeks, concluded that osteopenia of the distal femur and proximal tibia could be partly reversed through the use of FES.
There have also been very few studies involving pharmacotherapy.41, 42, 43, 44, 68, 69, 70 However, pharmacological intervention, particularly with bisphosphonates, appears to be a promising strategy. In a recent published study, the effects of long-term treatment with alendronate in a group of paraplegic men were evaluated in a prospective randomised controlled study.68 Bone loss was stoped at all cortical and trabecular infralesional sites over 24 months with alendronate 10 mg daily. Sniger and Garshick,69 also reported good results with the use of alendronate during 2 years in one patient with incomplete ASIA class D SCI. Calcitonin also represents an alternative, but further studies are needed in order to evaluate its role and benefits.70
Conclusions
Our results support the contention that bone loss can be safely attenuated in SCI patients through the use of antiresorptive therapy in the form of oral alendronate. Alendronate administration had a positive effect on BMD and therefore represents a potential tool for prevention and treatment of osteoporosis in this population. Prevention and treatment of reduced bone density is an important goal and is likely to prevent additional functional impairment and morbidity that might result in fracture. In order to justify the use of alendronate in SCI patients and analyse the cost–benefit relationship, further studies, including larger samples and longer follow-up periods, are warranted.
References
Demirel G, Yilmaz H, Paker N, Onel S . Osteoporosis after spinal cord injury. Spinal Cord 1998; 36: 822–825.
Lee TQ, Shapiro TA, Bell DM . Biomechanical properties of human tibias in long-term spinal cord injury. J Rehabil Res Dev 1997; 34: 295–302.
Szollar SM et al. Demineralization in tetraplegic and paraplegic man over time. Spinal Cord 1997; 35: 223–228.
Brito CM, Battistella LR, Sakamoto H, Sato ET . Bone mineral status after spinal cord injury. Acta Fisiátrica 2002; 9: 127–133.
Uebelhart D, Demiaux DB, Roth M, Chantraine A . Bone metabolism in spinal cord injured individuals and in others who have prolonged immobilisation. A review. Paraplegia 1995; 33: 669–673.
Roberts D et al. Longitudinal study of bone turnover after acute spinal cord injury. J Clin Endocriol Metab 1998; 83: 415–422.
Claus-Walker J, Halstead LS . Metabolic and endocrine changes in spinal cord injury: compounded neurologic dysfunctions. Arch Phys Med Rehabil 1982; 63: 632–638.
Leslie WD, Nance PW . Dissociated hip and spine demineralization: a specific finding in spinal cord injury. Arch Phys Med Rehabil 1993; 74: 960–964.
Garland DE et al. Osteoporosis after spinal cord injury. J Orthop Res 1992; 10: 371–378.
Kocina P . Body composition of spinal cord injury adults. Sports Med 1997; 23: 48–60.
Griffiths HJ, Bushuef B, Zimmerman RE . Investigation of the loss of bone mineral in patients with spinal cord injury. Paraplegia 1976; 14: 207–212.
Wilmet E et al. Longitudinal study of the bone mineral content and of soft tissue composition after spinal cord section. Paraplegia 1995; 33: 674–677.
Rodriguez GP, Claus WJ, Kent MC, Garza HM . Collagen metabolite excretion as a predictor of bone and skin related complications in spinal cord injury. Arch Phys Med Rehabil 1989; 70: 442–444.
Albright F, Burnett CH, Cope O, Parson W . Acute atrophy of bone (osteoporosis) simulating hyperparathyroidism. J Clin Endocrinol 1941; 1: 711–716.
Lazo MG et al. Osteoporosis and risk of fracture in men with spinal cord injury. Spinal Cord 2001; 39: 208–214.
Freehafer AA, Hazel CM, Becker CL . Lower extremity fractures in patients with spinal cord injury. Paraplegia 1981; 19: 367–372.
Barros Filho TE et al. Fratura de fêmur em pacientes portadores de lesão medular. Rev Hosp Clin Fac Med São Paulo 1991; 46: 289–292.
Keating JF, Kerr M, Delargy M . Minimal trauma causing fractures in patients with spinal cord injury. Disabil Rehabil 1992; 14: 108–109.
Hartkopp A et al. Bone fracture during electrical stimulation of the quadriceps in a spinal cord injured subject. Arch Phys Med Rehabil 1998; 79: 1133–1136.
Ragnarsson KT, Sell GH . Lower extremity fractures after spinal cord injury: a retrospective study. Arch Phys Med Rehab 1981; 62: 418–423.
Szollar SM . Osteoporosis in men with spinal cord injuries. West J Med 1997; 166: 270.
Szollar SM et al. Bone mineral density and indexes of bone metabolism in spinal cord injury. Am J Phys Med Rehabil 1998; 77: 28–35.
Frisbie JH . Fractures after myelopathy: the risk quantified. J Spinal Cord Med 1997; 20: 66–69.
Vestergaard P, Krough K, Rejnmark L, Mosekilde L . Fracture rates and risk factors for fractures in patients with spinal cord injury. Spinal Cord 1998; 36: 790–796.
Ingram RR, Suman RK, Freeman PAF . Lower limb fractures in the chronic spinal cord injured patient. Paraplegia 1989; 27: 133–139.
Kunkel CF et al. Effect of standing on spasticity, contracture, and osteoporosis in paralyzed males. Arch Phys Med Rehabil 1993; 74: 73–78.
Kaplan PE et al. Reduction of hypercalciuria in tetraplegia after weight bearing and strengthening exercises. Paraplegia 1981; 19: 289–293.
Saltzstein RJ, Hardin S, Hastings J . Osteoporosis in spinal cord injury: using an index of mobility and its relationship to bone density. J Am Paraplegia Soc 1992; 15: 232–234.
Goemaere S, Van Laere M, De Neve P, Kaufman JM . Bone mineral status in paraplegic patients who do or do not perform standing. Osteoporosis Int 1994; 4: 138–143.
de Bruin ED et al. Changes of tibia bone properties after spinal cord injury: effects of early intervention. Arch Phys Med Rehabil 1999; 80: 214–220.
Needham-Shropshire BM et al. Evaluation of a training program for persons with SCI paraplegia using the Parastep 1 ambulation system. Lack of effect on bone mineral density. Arch Phys Med Rehabil 1997; 78: 799–803.
Sipski ML et al. Prevention of osteoporosis through early use of electrical stimulation after spinal cord injury [abstract]. Arch Phys Med Rehabil 1990; 71: 795.
Mysiw J, Jackson R, Bloomfield S . Hypercalciuria prevented by functional electric stimulation [abstract]. Arch Phys Med Rehabil 1990; 7: 795.
Leeds EM et al. Bone mineral density after bicycle ergometry training. Arch Phys Med Rehabil 1990; 71: 207–209.
Mohr T et al. Increased bone mineral density after prolonged electrically induced cicle training of paralyzed limbs in spinal cord injured man. Calcif Tissue Int 1997; 61: 22–25.
BeDell KK, Scremin AME, Perell KL, Kunfel CF . Effects of functional electrical stimulation-induced lower extremity cycling on bone density of spinal cord injury patients. Am J Phys Med Rehabil 1996; 75: 29–34.
Bélanger M et al. Electrical stimulation: can it increase muscle strength and reverse osteopenia in spinal cord injured individuals? Arch Phys Med Rehabil 2000; 8: 1090–1098.
Greenspan SL et al. Bisphosphonates: safety and efficacy in treatment and prevention of osteoporosis. Am Fam Physician 2000; 61: 2731–2736.
Sankaran SK . Osteoporosis prevention and treatment. Pharmacological management and treatment implications. Drugs Aging 1996; 9: 472–477.
Pols HA et al. Effects of alendronate on BMO and fracture risk: The FOSIT Study. Osteoporosis Int 1999; 9: 461–468.
Pearson EG, Nance PW, Leslie WD, Ludwig S . Cyclical etidronate: its effect on bone density in patients with acute spinal cord injury. Arch Phys Med Rehabil 1997; 78: 269–272.
Minaire P et al. Effects of disodium dichloromethylene diphosphonate on bone loss in paraplegic patients. J Clin Invest 1981; 68: 1086–1092.
Minaire P et al. Effects of clodronate on immobilization bone loss. Bone 1987; 8(Suppl 1): 63–68.
Chappard D et al. Effects of tiludronate on bone loss in paraplegic patients. J Bone Miner Res 1995; 10: 112–118.
Maynard FM et al. International Standards for Neurological and Functional Classification of Spinal Cord Injury. American Spinal Injury Association: Chicago 1992.
Kirkwood BR . Essentials of Medical Statistics, 1st edn. Blackwell Science Publications: Oxford 1988.
Naftchi NE, Viau AT, Sell GH, Lowman EW . Mineral metabolism in spinal cord injury. Arch Phys Med Rehabil 1980; 61: 139–142.
Kearns PJ et al. Nutritional and metabolic response to acute spinal cord injury. J Parenter Enteral Nutr 1992; 16: 11–15.
Claus-Walker J, Halsted LS, Rodrigues GP, Henry YK . Spinal cord injury hypercalcemia: therapeutic profile. Arch Phys Med Rehabil 1982; 63: 108–115.
Maynard FM . Immobilization hypercalcemia following spinal cord injury. Arch Phys Med Rehabil 1986; 67: 41–44.
Bergmann P et al. Longitudinal study of calcium and bone metabolism in paraplegic patients. Paraplegia 1977–78; 15: 147–159.
Onhry A, Shemesh Y, Zak R, Herzberg M . Zinc and osteoporosis in patients with spinal cord injury. Paraplegia 1980; 18: 174–180.
Pietschmann P et al. Increased serum osteocalcin in patients with paraplegia. Paraplegia 1992; 30: 204–209.
Uebelhart D et al. Early modifications of biochemical markers of bone metabolism in spinal cord injury patients. A preliminary study. Scand J Rehabil Med 1994; 26: 197–202.
Varizi ND et al. Vitamin D, parathormone, and calcitonin profiles in persons with long-standing spinal cord injury. Arch Phys Med Rehabil 1994; 75: 766–769.
Stewart AF et al. Calcium homeostasis in immobilization an example of resorptive hypercalciuria. N Engl J Med 1982; 306: 1136–1139.
Finsen V, Intredavic B, Fougner KJ . Bone mineral and hormone status in paraplegics. Paraplegia 1992; 30: 343–347.
Biering-Sorensen F, Bohr H, Schaadt O . Bone mineral content of the lumbar spine and lower extremities years after spinal cord lesion. Paraplegia 1988; 26: 293–301.
Braga de Castro Machado A, Hannon R, Eastell R . Monitoring alendronate therapy for osteoporosis. J Bone Miner Res 1999; 14: 602–608.
Verheij LF et al. Optimization of follow-up measurements of bone mass. J Nucl Med 1992; 33: 1406–1410.
Pouilles JM, Tremollieres F, Todorovsky N, Ribot C . Precision and sensitivity of dual-energy X-ray absorptiometry in spinal osteoporosis. J Bone Miner Res 1991; 6: 997–1002.
Fuleihan GE et al. Reproducibility of DXA absorptiometry: a model for bone loss estimates. J Bone Miner Res 1995; 10: 1004–1014.
Johnson J, Damnson-Hughes B . Precision and stability of dual-energy X-ray absorptiometry measurements. Calcif Tissue Int 1991; 49: 174–178.
Haddaway MJ, Davie MW, McCall IW . Bone mineral density in healthy normal women and reproducibility of measurements in spine and hip using dual-energy absorptiometry. Br J Radiol 1992; 65: 213–217.
Liu CC et al. Quantitative computed tomography in the evaluation of spinal osteoporosis following spinal cord injury. Osteoporos Int 2000; 11: 889–896.
Rossini M et al. Long-term effects of a treatment course with oral alendronate of postmenoupausal osteoporosis. J Bone Miner Res 1994; 9: 1833–1837.
Adami S, Baroni MC, Broggini M, Carratelli L . Treatment of postmenopausal osteoporosis with continuos daily oral alendronate in comparison with either placebo or intranasal calcitonin. Osteoporosis Int 1993; 3(Suppl 3): S21–S27.
Zehnder Y et al. Prevention of bone loss in paraplegics over 2 years with alendronate. J Bone Miner Res 2004; 19: 1067–1074.
Sniger W, Garshick E . Alendronate increases bone density in chronic spinal cord injury: a case report. Arch Phys Med Rehabil 2002; 83: 139–140.
Carey DE, Raisz LG . Calcitonin therapy in prolonged immobilization hypercalcemia. Arch Phys Med Rehabil 1985; 66: 640–644.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Moran de Brito, C., Battistella, L., Saito, E. et al. Effect of alendronate on bone mineral density in spinal cord injury patients: a pilot study. Spinal Cord 43, 341–348 (2005). https://doi.org/10.1038/sj.sc.3101725
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.sc.3101725
Keywords
This article is cited by
-
Drug Repurposing for Spinal Cord Injury: Progress Towards Therapeutic Intervention for Primary Factors and Secondary Complications
Pharmaceutical Medicine (2023)
-
The efficacy and safety of bisphosphonate analogs for treatment of osteoporosis after spinal cord injury: a systematic review and meta-analysis of randomized controlled trials
Osteoporosis International (2021)
-
Open-label clinical trial of alendronate after teriparatide therapy in people with spinal cord injury and low bone mineral density
Spinal Cord (2019)
-
Osteoporosis prophylaxis in acute SCI
Spinal Cord Series and Cases (2019)
-
Evidence-based prevention and treatment of osteoporosis after spinal cord injury: a systematic review
European Spine Journal (2018)