Abstract
Study design: Pre–post training intervention.
Objectives: To evaluate the effect of training intensity on physical capacity, lipid profile and insulin sensitivity in early rehabilitation of spinal cord injured (SCI) patients, and to assess the correlation between peak aerobic capacity (VO2Peak) and insulin sensitivity.
Setting: Spinal Cord Rehabilitation Unit, Sunnaas Hospital, Nesoddtangen, Norway.
Method: Six recently injured SCI individuals participated in the arm training intervention and were randomly admitted to a high-intensity (HI; 70–80% heart rate reserve (HRR)) and low-intensity (LI; 40–50% HRR) group. The 1 h interval training consisted of 3 min exercise bouts interspersed with 2 min of rest, three times a week for 8 weeks. In addition, a correlation coefficient was obtained between VO2Peak and insulin sensitivity in 11 SCI patients.
Results: The 8-week training program resulted in a significant increase in VO2Peak and maximal power output (POMax) for the group as a whole (P<0.05). VO2Peak increased significantly more and total cholesterol/high-density lipoprotein cholesterol (TC/HDL-C) ratio and triglycerids decreased significantly more in the HI group than in the LI group (P=0.05). Training-induced changes in insulin sensitivity were significantly different between the groups (P=0.05), which was due to a nonsignificant decline in insulin sensitivity in the HI group and a nonsignificant improvement in the LI group. A significant positive correlation was found between VO2peak and insulin sensitivity (r=0.68, P=0.02).
Conclusion: The interval arm training protocol as used in the present study enables recently injured SCI patients to do substantial work at a relatively high intensity. Results indicate that improvements in physical capacity and lipid profile were more pronounced in response to high-intensity training. The significant correlation between maximal oxygen consumption and insulin sensitivity indicates that, as in the able-bodied population, peak aerobic capacity is a predictive value with regard to insulin sensitivity in SCI. Future studies with larger groups assessing the role of exercise intensity on insulin sensitivity in SCI are suggested.
Similar content being viewed by others
Introduction
A spinal cord injury (SCI) disrupts the nervous connections in the spinal cord and results in muscle paralysis, loss of sensation and autonomic dysfunction below the level of injury. In addition, well-known risk factors for cardiovascular diseases such as glucose intolerance, disturbances in lipid profile, alterations in body composition and morphological changes in skeletal muscles are observed in higher proportion in persons with long-term SCI.1,2,3,4,5,6 It is most likely that these profound metabolic alterations are related to the extreme physical inactivity, which is a direct consequence of the injury.7 Increasing physical activity may therefore play an important role in reducing these cardiovascular risk factors in SCI. In addition, physical exercise is needed if persons with SCI are to reach and maintain the level of physical fitness that is desirable for their function in daily life. Therefore, the effort to achieve optimal levels of physical fitness should start during primary rehabilitation itself. Numerous studies in able-bodied individuals have documented the health benefits of physical training.8,9,10 In the able-bodied population, health-related metabolic improvements are generally considered to be more pronounced in response to high-intensity exercise than to moderate to low-intensity exercise.11,12 However, only little is known about the most optimal training intensity in persons with SCI, and it is not known whether recently injured SCI persons can sustain a relatively high physical strain in early rehabilitation.
The use of arm exercise modes as used in SCI incorporates a relatively small muscle mass. The primary factors that limit performance during exercise may be peripheral in nature and local fatigue of the highly stressed arm musculature can occur despite sufficient systemic availability of blood and oxygen. Also, circulatory responses to exercise differ in spinal cord-injured individuals compared with able-bodied individuals as a result of a disturbed redistribution of blood.13,14 Maximal aerobic capacity (VO2Peak) is a predictive value with regard to insulin sensitivity in the able-bodied population, and a highly significant positive correlation between VO2Peak and insulin sensitivity has been reported.15,16 It is not known if there is also such a relationship in the spinal cord-injured population. The main purpose of the present study was to assess the effect of two different exercise intensities on insulin sensitivity, physical capacity and lipid profile during early rehabilitation in persons with SCI. In addition, a correlation coefficient was obtained between VO2peak and insulin sensitivity. To our knowledge, this is the first study to investigate these topics during the early phase following a SCI.
Methods
Subjects
Training intervention
Six SCI individuals (four males and two females) participated in the training intervention. All of them were wheelchair users. They were admitted for primary rehabilitation and their level of lesion varied from C5 to L1. Individual subject characteristics are shown in Table 2. Subjects known to have cardiovascular diseases and diabetes were excluded from the study. Group subject characteristics are presented in Table 1.
Correlation study
A total of 11 wheelchair bound SCI individuals admitted for primary rehabilitation were willing to participate in the correlation study. The level of lesion varied from C5 to L1. The same exclusion criteria as mentioned above were applied for the correlation study. Individual subject characteristics are presented in Table 2.
The study protocol was reviewed and approved by the Regional Ethics Committee and all subjects provided written, informed consent.
Design
Training intervention
The subjects were randomly admitted to a low-intensity (LI) or high-intensity (HI) training protocol. The training programs for both intensity groups consisted of a 1-h interval group training, three times a week for a period of 8 weeks. The different intensities were 70–80% of the heart rate reserve (HRR) in the HI group and 40–50% HRR in the LI group. This training intervention was conducted in addition to the usual daily rehabilitation program. Patients were evaluated on their physical work capacity as soon as they were able to sit in a wheelchair and carry out vigorous arm exercise. Insulin sensitivity and lipid profile were also determined during the same week. After 8 weeks of training, all subjects were retested at the same point of time according to the same protocol as used in the pretest.
Correlation study
All subjects underwent an insulin sensitivity test followed by an exercise test in the same week. Pretraining measurements of VO2Peak and insulin sensitivity from the six subjects participating in the intervention study were also used for analyses in the correlation study.
Test protocol
Physical capacity
The arm exercise test consisted of a discontinuous graded exercise protocol using a calibrated ergometer 800 (Ergoline, Germany). Generally, 3 min of arm cranking were separated by 2 min of rest. Blood pressure was measured (9300, Paramed) immediately after each exercise bout. The power output was increased with equal steps. The average power output at the start was 16 W with a standard deviation of 7 W. The cycling rate had to be kept constant in the range between 60 and 70 rotations per minute. The exercise test was ended when the subject could no longer sustain the pace in spite of verbal encouragement. Heart rate (HR) and power output (PO watts) were measured continuously. Oxygen uptake (VO2, l/min and ml/kg/min) was measured breath-to-breath during the exercise test and the highest value averaged over 30 s was taken as VO2Peak (Vmax 229, Sensormedics).
Insulin sensitivity
The HOMA-CIGMA test17,18 was used to measure insulin sensitivity. Subjects were instructed to refrain from exercise for 48 h and they were tested after an overnight fast in supine position. Intravenous catheters were inserted into elbow veins in both arms. Three blood samples were drawn at -15, -10 and -5 min through a Venflon catheter (1.2 × 32 mm, Becton Dickinson) for glucose and insulin analyses. At 0 min, subjects were administered to a continuous intravenous infusion of 5 mg glucose/kg ideal body weight/min during 1 h (Metropolitan Height-Weight Tables, 1983). At 50, 55 and 60 min after the start of the infusion, three venous blood samples were withdrawn for analyses of glucose and insulin. Blood glucose concentration was determined directly after sampling (Vitros 250, Johnson & Johnson, Ortho Clinical Diagnostics). Blood plasma samples were frozen (−30°C) for further insulin analyses. Insulin was measured using a competitive RIA (Linco Research Inc. St Charles, MO, USA).
Lipid profile
From fasting blood samples, total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C) and triglycerides (TG) were obtained (Vitros 250, Johnson & Johnson, Ortho Clinical Diagnostics). Low-density lipoprotein cholesterol (LDL-C) was calculated by Friedewald's formula.19
Training session
For the HI and the LI groups, the 1-h training sessions contained the same protocol, only the intensities differed. The sessions aimed at maintaining the HR between 50–60 or 70–80% of HRR,20 for 20 min.

The resting heart rate was measured during rest early in the morning on one occasion before the training program had started. The peak heart rate was derived from the pretest peak exercise arm-crank test. The training consisted of arm-cranking, in intervals of 3 min cranking and 2 min of rest. In order to prevent the training from becoming monotonous, the arm-cranking was interchanged with exercises such as boxing, push-ups and ball throwing. At all sessions, the heart rate was monitored with Polar sport tester.
Statistics
Statistical analyses were performed using SPSS 8.0. The heterogeneity of the groups and the small number of subjects demanded that nonparametric statistics be used. Owing to the large interpersonal differences, data in the intervention study were taken relative to the initial state ((post-test values/pretest values) × 100%). A Wilcoxon-signed rank test was used to compare pre- and post-training values. Differences between HI and the LI group were compared using a Mann–Whitney U-test. To study the association between the insulin sensitivity and peak aerobic capacity, a correlation analysis was carried out with a Spearman rank-difference correlation. The level of significance for all tests was at P-value 0.05.
Results
Subject characteristics like body mass, body height and time since injury did not differ between the groups. Age was significantly lower in the HI group. The results of pretraining measurements on physical capacity, lipid profile and insulin sensitivity did not differ between the groups.
Training intervention
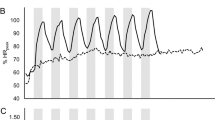
The 8-week training program resulted in a significant increase in VO2Peak and POMax for the group as a whole (P<0.05). The individual absolute results on VO2Peak, POMax, insulin sensitivity and lipid profile parameters are presented in Figure 1. Post-test values relative to pre-test values of both intensity groups are presented in Table 3. It is shown that VO2Peak increased significantly more, and triglycerides and TC/HDL ratio decreased significantly more in the HI group than in the LI group (P=0.05). Furthermore, results show a significant difference between the groups for insulin sensitivity (P=0.05), which was due to a nonsignificant decrease in insulin sensitivity in the HI group and a nonsignificant improvement in the LI group. The increase in POMax and the changes in lipid profile parameters TC, HDL and LDL did not differ between the two groups.
Individual absolute pre- and post-test values for VO2Peak, power output (PO), insulin sensitivity (IS), ratio total cholesterol/high-density lipoprotein cholesterol (TC/HDL-C), TC, HDL-C, low-density lipoprotein cholesterol (LDL-C) and triglycerides (TG). HI: high-intensity group. LI: low-intensity group. ▪ Pre-Training HI ▪ Pre-Training LI □ Post-Training HI □ Post-Training LI.
Correlation study
The Spearman correlation coefficient revealed a significant correlation between VO2Peak and insulin sensitivity (r=0.68; P=0.02; Figure 2).
Discussion
In the present study, we evaluated the effect of training intensity on physical capacity, lipid profile and insulin sensitivity in early rehabilitation of SCI individuals. In addition, a group of SCI individuals in primary rehabilitation was studied at a single time point to assess the correlation between peak aerobic capacity (VO2Peak) and insulin sensitivity.
Physical capacity
Results of the present study show that 8 weeks of arm exercise training induces a significant increase in cardiopulmonary fitness (VO2Peak) and maximal power output (POMax) for the group as a whole. Improvements in VO2Peak were more pronounced in response to HI training, that is, post-training levels were 150% for the HI group and 117% in the LI group. In addition, a trend towards a greater increase in POMax was found for the HI group (159 versus 124%). These findings in our fairly recently injured SCI individuals are in line with previous studies assessing the effect of arm training intensity on physical capacity in able-bodied subjects21 and long-term spinal cord-injured individuals.22
Lipid profile
Evidence suggests that cardiovascular diseases, particularly coronary heart disease (CHD), are the leading cause of death in the chronic spinal cord injured population.23 Previous studies reported unfavourable lipid and (apo) lipoprotein profiles in persons with SCI.4,7 It has been suggested that the low levels of HDL-C concentrations are the consequence of the extremely low levels of physical activity in the SCI population, mainly as a result of their wheelchair-dependent life.2,7 Indeed, earlier studies have demonstrated that highly trained SCI subjects had more favourable lipid profiles than untrained SCI subjects, indicating an important role for physical activity.4 In the present study, it was found that the ratio TC/HDL-C, which is considered to be a stronger predictor of CHD than HDL-C alone,24 and TG decreased significantly more in the HI group than in the LI group. Although a positive trend can be seen, the changes in TC, HDL-C and LDL-C did not differ between the two intensity groups. Findings of the present study in relatively acute SCI individuals are in agreement with a previous study assessing the effect of low (50–60% HRR) and moderate training intensity (70–80% HRR) on lipid profile in chronic SCI individuals.22 The authors demonstrated that blood lipid levels remained unaltered in the LI group, while the moderate-intensity group exhibited significant increases in HDL-C and decreases in TG, LDL-C and TC/HDL-C ratio.
Insulin sensitivity
Chronic SCI individuals are characterized by alterations in body composition and muscle morphology, glucose intolerance and a decreased whole-body insulin sensitivity.5,25,26 These metabolic disturbances are thought to contribute to the increased risk for cardiovascular diseases (CVD) in this group. Numerous previous investigations have shown the beneficial effects of physical training on insulin sensitivity in both healthy and glucose-intolerant subjects.8 Hjeltnes et al27 assessed the effect of 8 weeks of electrically stimulated leg cycling (ESLC) in chronic tetraplegics and reported an increase in whole-body insulin sensitivity and an increase in protein expression of key genes involved in glucose metabolism. In agreement with these findings, other recent studies in chronic SCI individuals demonstrated similar beneficial results of electrically induced cycle training on insulin sensitivity and glucose tolerance.28,29
In our study, the HOMA-CIGMA test was used to assess insulin sensitivity. This model has been shown to be a reliable, easy, quick and low-cost method, has a good test–retest reliability and can be used to detect changes on the individual level.17,30 Findings of the present study indicate that the arm exercise training induced a significant difference in insulin sensitivity between the LI group and the HI group. In contrast to what we expected, this difference between the groups was due to a nonsignificant decrease in insulin sensitivity in the HI group and a nonsignificant increase in insulin sensitivity in the LI group. These results are contrary to the accepted dogma in the literature and not in agreement with a previous study assessing the role of exercise intensity on insulin sensitivity in a group of 18 healthy able-bodied subjects.12 Borghouts et al reported that improvements in insulin sensitivity were more pronounced in response to 4 weeks of HI interval training than to LI interval training. Their training protocol consisted of 3 min exercise bouts interspersed with 2 min at a lower intensity on a bicycle ergometer, with intensities 80–40% of pre-training maximal power output (Wmax) in the HI group and 40–20% Wmax in the LI group.12
It seems evident that results of our study must be viewed with cautiouns due to several factors. First of all, the small sample size in the present study with only three subjects per intervention group is obviously too small to draw firm conclusions. Furthermore, subjects in the intervention differed in age, sex, level and completeness of injury, which may all influence the results. In addition, in the early phase after an SCI, there are several variables that might affect insulin sensitivity by release of counter regulatory hormones. Although the differences in pretraining values of insulin sensitivity were not significant, it seems that the HI group had the most insulin-sensitive individuals at baseline (HI:103±46 versus LI: 48±18%). After the 8-week training program, both groups had mean insulin sensitivity values that were nearly similar (HI: 66±27 versus LI:74±42%), which may indicate a sort of natural ‘regression towards a mean’ effect for both groups. It may be hypothesized that arm exercise, where only a limited muscle mass can be used, may not be sufficient to elicit changes in glucose homeostasis. However, the role of exercise intensity in SCI individuals is not clear and needs to be restudied in further studies with larger groups.
All six subjects completed the 8-week arm exercise training period (92% participation of training sessions) and they were able to maintain the heart rate at the prescribed intensity of 40–50% HRR or 70–80% HRR. At present, relatively few studies have been conducted on the role of exercise intensity in patient groups. This is probably due to the fact that patients are usually unable to perform prolonged HI training and the greater risk of health complications and injuries. The interval training protocol as used in the present study enabled the patients to do substantial work at a relatively HI. Since the expansion of physical capacity and improvement of the lipid profile seems to be more pronounced in response to HI training, HI training seems to be preferable when the training is supervised and compatible with the fitness status of the patient.
Correlation study
Eight out of 11 wheelchair-bound SCI subjects participating in the correlation study show a insulin sensitivity value below 100%, indicating a state of insulin resistance, in the early phase after the injury it self. In the present study, a significant correlation was found between insulin sensitivity (mean value 84±37%) and peak oxygen uptake (1.09±0.35 l/min). This is in agreement with previous studies in able-bodied populations where a highly positive correlation between insulin sensitivity and maximal oxygen consumption has been reported.15,16,31 The present results indicate that, as in the able-bodied population, peak aerobic capacity seem to be a predictor of insulin sensitivity in spinal cord-injured individuals. Metabolic disturbances such as a decreased insulin sensitivity are considered to play an important role in the increased risk for cardiovascular diseases In this group. Therefore, regular aerobic fitness assessment in SCI individuals may be of importance in the CVD prevention planning for the SCI.
Limitations of the study
It is clear that the present study has several limitations that must be taken into consideration. First of all, the study was performed in fairly recently injured spinal cord-injured individuals in a primary rehabilitation setting where changes might be influenced by other factors like the usual rehabilitation program and natural processes of adaptations. Furthermore, no control group and only a small and heterogeneous group of subjects was included. They differed in sex, age, level and completeness of injury that all may have an effect on the results. However, we took post-test values relative to the initial values in order to overcome the large intersubject differences. Despite the evident limitations of the present study, it is of clinical relevance to find the most optimal treatment in SCI in the early rehabilitation phase itself. With early activation and mobilization, it might be able to avoid profound metabolic alterations, thereby decreasing cardiovascular risk factors and achieving optimal physical fitness that is desirable for functioning in daily life.
In conclusion, the use of an arm exercise interval training protocol as used in the present study enables recently injured SCI patients to do substantial work at a relatively HI. Improvements in physical capacity and lipid profile were more pronounced in response to high training intensity. A significant positive correlation was found between maximal oxygen consumption and insulin sensitivity, indicating that, as in the able-bodied population, the peak aerobic capacity is a predictive value with regard to insulin sensitivity in SCI. Further studies with larger groups assessing the effect of exercise on insulin sensitivity in the spinal cord population are recommended.
References
Bauman WA et al. Depressed serum high density lipoprotein cholesterol levels in veterans with spinal cord injury. Paraplegia 1992; 30: 697–703.
Bauman WA et al. Coronary artery disease: metabolic risk factors and latent disease in individuals with paraplegia. Mt Sinai J Med 1992; 59: 163–168.
Bauman WA et al. The effect of residual neurological deficit on serum lipoproteins in individuals with chronic spinal cord injury. Spinal Cord 1998; 36: 13–17.
Dallmeijer AJ, Hopman MT, van der Woude LH . Lipid, lipoprotein, and apolipoprotein profiles in active and sedentary men with tetraplegia. Arch Phys Med Rehabil 1997; 78: 1173–1176.
Hjeltnes N et al. Improved body composition after 8 wk of electrically stimulated leg cycling in tetraplegic patients. Am J Physiol 1997; 273: R1072–R1079.
LaPorte RE et al. HDL cholesterol across a spectrum of physical activity from quadriplegia to marathon running. Lancet 1983; 1: 1212–1213.
Dearwater SR et al. Activity in the spinal cord-injured patient: an epidemiologic analysis of metabolic parameters. Med Sci Sports Exerc 1986; 18: 541–544.
Dela F et al. Effect of training on insulin-mediated glucose uptake in human muscle. Am J Physiol 1992; 263 (6 Part 1): E1134–E1143.
Tran ZV, Weltman A, Glass GV, Mood DP . The effects of exercise on blood lipids and lipoproteins: a meta-analysis of studies. Med Sci Sports Exerc 1983; 15: 393–402.
Mikines KJ et al. Effect of physical exercise on sensitivity and responsiveness to insulin in humans. Am J Physiol 1988; 254 (3 Part 1): E248–E259.
Tremblay A, Simoneau JA, Bouchard C . Impact of exercise intensity on body fatness and skeletal muscle metabolism. Metabolism 1994; 43: 814–818.
Borghouts LB, Backx K, Mensink MF, Keizer HA . Effect of training intensity on insulin sensitivity as evaluated by insulin tolerance test. Eur J Appl Physiol Occup Physiol 1999; 80: 461–466.
Hopman MT et al. Blood redistribution and circulatory responses to submaximal arm exercise in persons with spinal cord injury. Scand J Rehabil Med 1998; 30: 167–174.
Hopman MT et al. Limits to maximal performance in individuals with spinal cord injury. Int J Sports Med 1998; 19: 98–103.
Horton ES . Exercise and physical training: effects on insulin sensitivity and glucose metabolism. Diabetes Metab Rev 1986; 2: 1–17.
Koivisto VA, Yki-Jarvinen H, DeFronzo RA . Physical training and insulin sensitivity. Diabetes Metab Rev 1986; 1: 445–481.
Matthews DR et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985; 28: 412–419.
Hosker JP et al. Continuous infusion of glucose with model assessment: measurement of insulin resistance and beta-cell function in man. Diabetologia 1985; 28: 401–411.
Friedewald WT, Levy RI, Fredrickson DS . Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972; 18: 499–502.
Wilmore JHC . Physiology of Sports and Exercise: Human Kinetics: Champaign, 1994.
van der Woude LH et al. Physical work capacity after 7 wk of wheelchair training: effect of intensity in able-bodied subjects. Med Sci Sports Exerc 1999; 31: 331–341.
Hooker SP, Wells CL . Effects of low- and moderate-intensity training in spinal cord-injured persons. Med Sci Sports Exerc 1989; 21: 18–22.
Whiteneck GG et al. Mortality, morbidity, and psychosocial outcomes of persons spinal cord injured more than 20 years ago. Paraplegia 1992; 30: 617–630.
Stampfer MJ et al. A prospective study of cholesterol, apolipoproteins, and the risk of myocardial infarction. N Engl J Med 1991; 325: 373–381.
Duckworth WC et al. Glucose intolerance due to insulin resistance in patients with spinal cord injuries. Diabetes 1980; 29: 906–910.
Aksnes AK et al. Intact glucose transport in morphologically altered denervated skeletal muscle from quadriplegic patients. Am J Physiol 1996; 271 (3 Part 1): E593–E600.
Hjeltnes N et al. Exercise-induced overexpression of key regulatory proteins involved in glucose uptake and metabolism in tetraplegic persons: molecular mechanism for improved glucose homeostasis. FASEB J 1998; 12: 1701–1712.
Mohr T et al. Insulin action and long term-electrically induced training in individuals with spinal cord injuries. Med Sci Spo Exer 2001; 33: 1247–1252.
Jeon J et al. Improved glucose tolerance and insulin sensitivity after electrical stimulation-assisted cycling in people with spinal cord injury. Spinal Cord 2002; 40: 110–117.
Hermans MP, Levy JC, Morris RJ, Turner RC . Comparison of insulin sensitivity tests across a range of glucose tolerance from normal to diabetes. Diabetologia 1999; 42: 678–687.
Ebeling P et al. Mechanism of enhanced insulin sensitivity in athletes. Increased blood flow, muscle glucose transport protein (GLUT-4) concentration, and glycogen synthase activity. J Clin Invest 1993; 92: 1623–1631.
Acknowledgements
The enthusiastic participation of all subjects in the study is greatly appreciated. We acknowledge Wenche Ronning and other employees of the Clinical Chemical Laboratory at Sunnaas Hospital for their assistance with the measurements and analyses of blood samples.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
de Groot, P., Hjeltnes, N., Heijboer, A. et al. Effect of training intensity on physical capacity, lipid profile and insulin sensitivity in early rehabilitation of spinal cord injured individuals. Spinal Cord 41, 673–679 (2003). https://doi.org/10.1038/sj.sc.3101534
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.sc.3101534
Keywords
This article is cited by
-
Hybrid high-intensity interval training using functional electrical stimulation leg cycling and arm ski ergometer for people with spinal cord injuries: a feasibility study
Pilot and Feasibility Studies (2022)
-
Physiological responses to moderate intensity continuous and high-intensity interval exercise in persons with paraplegia
Spinal Cord (2021)
-
The effectiveness of vigorous training on cardiorespiratory fitness in persons with spinal cord injury: a systematic review and meta-analysis
Spinal Cord (2021)
-
Viability of high intensity interval training in persons with spinal cord injury—a perspective review
Spinal Cord (2021)
-
A tele-health intervention to increase physical fitness in people with spinal cord injury and cardiometabolic disease or risk factors: a pilot randomized controlled trial
Spinal Cord (2021)