Abstract
Background: Survival following spinal cord injury (SCI) has greatly improved since the unsuccessful attempts to repair the damaged spinal cord were replaced by systematic prevention and treatment of complications caused by the neural damage.
Objective: To evaluate the main outcome measures in patients with spinal cord injury.
Design: Retrospective cohort study.
Setting: Loewenstein Rehabilitation Hospital, the major referral center for rehabilitation medicine for hospitals throughout Israel.
Subjects: 250 consecutive patients, injured between 1959 and 1992.
Main outcome measures: Survival rates and mortality risk factors.
Method: Demographic, clinical, and mortality data were collected from the hospital charts and from the Population Registry of the Israel Ministry of Internal Affairs. Survival rates were estimated using the product limit (Kaplan-Meyer) method, and their association with known risk factors was analyzed with the Cox proportional hazard model.
Results: The survival rate after injury was 81% after 10 years, 75% after 20 years, and 62% after 30 years, and 50% after about 36.5 years. Survival was found to be negatively associated with age (P=0.01) and with high spinal level of injury (P=0.003).
Conclusions: Survival rates in the studied population are similar to those reported in other countries, and are close to those of the general population living in Israel in the same time period. The study demonstrates that developing countries can reach survival rates comparable to those of developed countries, and may contribute to better survival predictions of patients with SCI.
Similar content being viewed by others
Introduction
Until the last few decades, spinal cord injury (SCI) was associated with poor survival. In World War I, 80% of American and British soldiers who sustained SCI died within a few weeks to 3 years.1 The tide turned with the introduction of a new medical approach based on the notion that morbidity and mortality associated with SCI were due not to the neurological deficit per se but to its complications. Survival rates greatly improved when attempts to repair the damaged spinal cord were replaced with the systematic prevention and treatment of complications.2,3,4,5,67,8,9,10,11,12,13,14,15,16,17
Survival analysis of patients with SCI is important both for epidemiological description needed to assess and compare treatment achievements, and for estimating the survival of populations and of individual patients. Demonstrating the efficiency of treatment and predicting the survival rate of current patients may affect survival rates in the future by influencing resource allocation throughout the health system in general and for individual patients.
The survival, life expectancy, and mortality of patients with SCI have been discussed in many publications, and the information required for a positive influence on the survival of these patients is apparently available. Nevertheless, although the existing literature supports the current approach to the treatment and care of this population, the systematic prevention and treatment of complications is still partial, especially in developing countries and even in parts of developed ones. Moreover, many of the studies are not helpful for estimating survival in actual groups or individuals; their data are based on patients from specific countries, areas, or health systems, and does not easily lend itself to inferences on survival in other parts of the world. Some studies include only mortality data and lack information that can be easily interpreted for survival prediction.
Therefore, to support the current approach to care and treatment, and to improve the ability to predict the survival of patients with SCI, additional data are needed on survival (rather than mortality) rates of patients with SCI, in different subpopulations, cultures, areas, and countries.
The present study, which evaluates the survival rates and mortality risk factors in patients with traumatic spinal cord lesions, is the contribution of one center in Israel to achieving this purpose.
Methods
The study sample included 250 consecutive patients with traumatic spinal cord lesion, injured between 1959 and 1992 and treated at the Loewenstein Rehabilitation Hospital, the major referral center for rehabilitation medicine for hospitals throughout Israel. The number of admissions was distributed almost equally over time, with 74 patients admitted during the 1960s, 76 during the 1970s, 73 during the 1980s, and 27 in the early 1990s. Demographic and clinical data were collected by reviewing the hospital charts. Mortality data were collected from the Population Registry of the Israel Ministry of Internal Affairs. The study was carried out between July and December 1998. Mortality data through September 30, 1998 were used, so the time from the onset of the spinal cord injury to death or end of follow-up covered a maximum period of 39 years. The follow up period was 6 weeks to 10 years for 87 patients, 10–20 years for 61 patients, 20–30 years for 69 patients, and 30–39 years for 33 patients.
Data were analyzed by the SAS system for Windows, version 6.12 (SAS Institute, Cary, NC, USA). Survival rates were estimated using the product limit (Kaplan-Meyer) method, and differences between subgroups (by sex, age at injury, spinal level of injury, severity of neurological deficit, and decade of injury) were analyzed by logrank test. The Cox proportional hazard model was employed to determine the probability of mortality (`hazard') in the presence of specific risk factors and to create survival curves for various combinations of age and level of injury (Figure 1).18 The severity of neurological deficit below the spinal level of injury was graded according to Frankel.19
Results
Demographic and clinical data
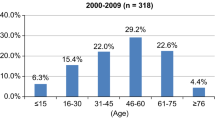
The age range of the patients was 6–83 years (mean 34.5). Male : female ratio was 3.1 : 1. The interval from injury to transfer to the rehabilitation center from a general hospital ranged from 3 to 384 days (mean 59, median 36). Levels of injury were as follows: high cervical, 7.6%; low cervical, 28.8%; thoracic, 32.4%; lumbar, 31.2%. On admission, the cord injury was grade A (complete) in 29.6%, grade B in 16.8%, grade C in 40%, and grade D in 13.6%. None of the patients was ventilator dependent. The cause of injury was road accident in 32.8%, work accident in 26.8%, a fall from a height (for unknown reasons) in 16.8%, suicide attempt in 13.6%, and other (gunshot, stabbing, sports-related accidents) in 10.4%.
Outcome
Maximum survival was 39 years at the time of data conclusion (43 years by the year 2002); 181 of the 250 patients were alive at the end of the follow-up period. Of the remainder, 12 patients died in the first year after injury, eight during the second year, four during the third, four during the fourth and six during the fifth year; all together 34 within 5 years. 0–3 patients died during each of the subsequent years.
The 5-year survival rate was 85.2%, and the 30 years survival rate, 62.24% (Table 1). Survival rate was less than 50% only for the patients who were followed for at least 35 years (36.5 years on average).
Survival was found to be negatively associated with age at injury (P=0.001) and with high spinal level of injury (P=0.003). It was also associated negatively with severity of neurological deficit and positively with recency of injury (by decade), but neither of these findings reached statistical significance. There was no significant association between gender and survival. Grouping the patients by age and level of lesion, survival rate was 50% at 11 years after the injury, and 20% at 30 years after the injury in patients with cervical lesions who were injured when they were aged 35 years or older. In the patients with thoracic lesions who were injured when they were aged 35 or older, survival rate was 50% at 16 years after injury and 30% at 30 years after the injury. In patients with lumbar lesions who were injured when they were aged 35 years or older, survival rate was over 65% at 30 years after injury. In the patients with cervical lesions who were injured when they were aged 34 years or younger, survival rate was 70% at 30 years after injury. In the patients with thoracic lesions who were injured when they were aged 34 years or younger, survival rate was approximately 80% at 30 years after the injury. In the patients with lumbar lesions who were injured when they were aged 34 years or younger, survival rate was over 90% at 30 years after injury (Figure 1).
Out of the 35 patients with cervical lesions and a Frankel grade of A or B on admission, who survived the first year after injury, three are still alive 34–43 years after the injury.
Discussion
Loewenstein Rehabilitation Hospital opened in 1962 and admitted patients who had sustained spinal injuries from 1959 onwards. Most of the patients were transferred from a general hospital, and many had undergone spinal surgery before rehabilitation. The treatment applied at the Loewenstein Rehabilitation Hospital followed the principles established by Guttmann.1 Admission was followed by routine check-up examinations performed during short hospitalizations. The present study evaluates the success of this approach in terms of survival.
In general, the survival rates found in this study represent a satisfactory achievement of the treatment and are similar to those of corresponding large-scale studies in western countries.11,12 About half the patients with sufficient follow-up were still alive at 36.5 years after injury, a finding comparable to the 33-median years of survival described by Whiteneck et al.12 Adding the mean age of the patients, 24.5 years to the median survival time, results in an average life span of approximately 71 years for the patient population. This is slightly less than that for the general hospital population of Israel during the same period. These results demonstrate that developing countries can reach survival rates comparable to those of developed countries by adopting the principles introduced into SCI medicine in the second half of the twentieth century.1 Applying the conclusions of this and other corresponding large-scale studies can improve the lifetime compensation offered to SCI patients in Israel and other countries with similar characteristics. The results confirm the reference to the median of subgroups, as presents here and in previous publications, and at the same time encourage the extrapolation of estimated survival (and compatible compensation) according to specific patient characteristics. Characteristics such as younger age at injury, lower level and degree and severity of the lesion, more recent date of onset of injury, and a longer interval between injury and estimation date, should prolong the estimated survival. Our finding that approximately half the mortality (34 out of 69) occurred during the first 5 years after injury indicates that survival of 5 years or longer predicts a long life expectancy. The overall favorable outcome of the treatment and long-term follow-up at a specific medical center may support the reference to the availability of specialized services in the process of estimating the survival of patients with SCI.
The negative association of survival with age at injury and level of lesion agrees with many previous studies.4,8,12,16 The study also found an association, albeit not statistically significant, between survival and both severity of neurological deficit and recency of injury. Similarly, Sarnsa et al13 failed to demonstrate a significant association between survival and severity of neurological deficit, and Measard et al3 found such an association only when the level of injury was cervical or high thoracic. However, a statistically significant association between survival and severity of neurological deficit was found by DeVivo et al.16 Conclusions regarding the influence of gender on survival after cord injury are likewise conflicting. Some studies have demonstrated a significantly longer survival rate among females,6,15,16 whereas others,13 including the present one, have not. Despite its generally positive findings, the study has several limitations. The number of participants (n=250) was too small for actuarial purposes. Insufficient follow-up in many of them did not allow accurate calculations of life expectancies, especially by age, lesion level, and other factors. In addition, differences in the interval from injury to transfer to rehabilitation, era of injury, and inclusion of first-year deaths, made comparison with other studies difficult. The relatively long interval between injury and admission in the present study (median 36 days) results in the exclusion of early deaths, thereby reducing the first-year mortality and increasing the survival rate relative to some earlier studies.4,8 At the same time, the inclusion of all other first-year facilities decreased the survival rate relative to other important studies.12,16
In conclusion, these results resemble in general those of recent studies elsewhere, and survival rates are close to those of the general Israeli population during the same time period. These findings confirm the modern approach to the management of SCI and contribute to more accurate predictions of survival for patients with SCI.
References
Guttmann L . Spinal Cord Injuries: Comprehensive Management and Research Oxford: Blackwell 1976
Geisler WO, Jousse AT, Wynne-Jones M . Survival in traumatic transverse myelitis Paraplegia 1977 14: 262–275
Measard L, Carmody A, Mannarino E, Ruge D . Survival after spinal cord trauma; a life table analysis Arch Neurol 1978 35: 78–83
DeVivo JM, Fine RF, Maetz HM, Stover SL . Prevalence of spinal cord injury Arch Neurol 1980 37: 707–708
Minaire P et al. Life expectancy following spinal cord injury: A ten-year survey in the Rhone-Alpes region, France, 1969–1980 Paraplegia 1983 21: 11–15
Griffin HR et al. Mortality, survival and prevalences of traumatic spinal cord injury in Olmstead County, Minnesota, 1935–1981 J Chron Dis 1985 38: 643–653
DeVivo JM et al. Seven-year survival following spinal cord injury Arch Neurol 1987 44: 872–875
DeVivo JM, Stover SL, Black KJ . Prognostic factors for 12-year survival after spinal cord injury Arch Phys Med Rehabil 1992 73: 156–162
DeVivo JM et al. Trends in spinal cord injury demographics and treatment outcomes between 1973 and 1986 Arch Phys Med Rehabil 1992 73: 424–430
DeVivo JM, Black KJ, Stover SL . Causes of death during the first 12 years after spinal cord injury Arch Phys Med Rehabil 1992 74: 248–254
Krause JS, Kjorsving JM . Mortality after spinal cord injury: A four-year prospective study Arch Phys Med Rehabil 1922 73: 558–563
Whiteneck et al. Mortality, morbidity, and psychological outcomes of persons spinal cord injured more than 20 years ago Paraplegia 1992 30: 617–630
Sarnsa GP, Patrick CH, Feussner JR . Long-term survival of veterans with traumatic spinal cord injury Arch Neurol 1993 50: 909–914
Hartkopp A, Bronnum-Hansen H, Seidenschnur AM, Biering-Sorensen F . Survival and cause of death after traumatic spinal cord injury. A long-term epidemiological survey from Denmark Spinal Cord 1997 35: 76–85
Frankel HL et al. Long-term survival in spinal cord injury: a fifty-year investigation Spinal Cord 1998 36: 266–274
DeVivo MJ, Krause JS, Lammertse DP . Recent trends in mortality and causes of death among persons with spinal cord injury Arch Phys Med Rehabil 1999 80: 1411–1419
Zeilig G, Dolev M, Weingarden H, Blumen N, Shemesh Y, Ohry A . Long-term morbidity and mortality after spinal cord injury: 50 years of follow-up Spinal Cord 2000 38: 563–566
Dawson-Saunders B, Trapp RG . ed Methods for analysing survival data In: Basic and Clinical Biostatistics Norwalk, Connecticut: Appleton and Lange 1994 pp 188–209
Frankel HL et al. The value of postural reduction in the initial management of closed injuries of the spine with paraplegia and tetraplegia Paraplegia 1969 24: 179–192
Acknowledgements
The authors express their special gratitude to Dr L Mendelson, Medical Director of Spinal Rehabilitation of Loewenstein Hospital until 1992. This study was supported by the Unit of Medical Services, Rehabilitation Department, Israel Ministry of Defense.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Catz, A., Thaleisnik, M., Fishel, B. et al. Survival following spinal cord injury in Israel. Spinal Cord 40, 595–598 (2002). https://doi.org/10.1038/sj.sc.3101391
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.sc.3101391
Keywords
This article is cited by
-
A prediction model to identify people with spinal cord injury who are at high risk of dying within 5 years of discharge from hospital in Bangladesh
Spinal Cord (2019)
-
Factors predictive of survival and estimated years of life lost in the decade following nontraumatic and traumatic spinal cord injury
Spinal Cord (2017)
-
Long-term survival after traumatic spinal cord injury: a 70-year British study
Spinal Cord (2017)
-
Incidence of traumatic spinal cord injury worldwide: a systematic review
European Spine Journal (2015)
-
Burden of spinal cord injury in Tehran, Iran
Spinal Cord (2010)