Abstract
Study design: Case report.
Objective: To illustrate the significance of vertebral body infarction as the only confirmatory sign of spinal cord ischemic stroke.
Setting: Department of Neurology, St. Josef-Hospital, Bochum, Germany.
Case description: A 65-year-old man presented with clinical features suggesting spontaneous spinal cord infarction in the territory of the left sulcocommissural artery at the level of Th 8. Sequential magnetic resonance imaging (MRI) investigations failed to demonstrate infarction of the spinal cord but T2-weighted images revealed bone marrow hyperintensities of vertebra Th 9 and 10 in their left and dorsal parts consistent with vertebral body infarction.
Conclusion: In clinically presumed spontaneous spinal cord infarction and unremarkable signaling of the spinal cord during sequential MRI investigations vertebral body infarction may serve as the only confirmatory sign of spinal cord ischemic stroke.
Similar content being viewed by others
Introduction
In contrast to the frequent occurrence of cerebral infarction spinal cord ischemic stroke is uncommon and in most cases not spontaneous.1,2 In clinically presumed spinal cord infarction, diagnostic procedures may be inconclusive despite extensive efforts. Previous reports have pointed out the significance of corresponding vertebral body infarction revealed by serial magnetic resonance imaging (MRI) confirming the vascular nature of acute spinal cord syndromes.3,4,5,6 We describe a patient who presented with clinical features suggesting infarction in the territory of the left sulcocommisural artery at the level of Th 8. Sequential MRI failed to demonstrate infarction of the spinal cord but showed corresponding bone marrow hyperintensities in the vertebral column depicting vertebral body infarction as the only confirmatory sign of midthoracic spinal stroke.
Case Report
On December 23, 1999, a 65-year-old man with a history of smoking, hypertension, insulin-dependent diabetes mellitus and coronary bypass operation was admitted to our hospital. He complained of a weakness of his left leg and a left-sided squeezing pain at the level of the hypochondriac regions since the day before.
On examination he presented with paresis of the left abdominal musculature and a mild flaccid paresis of the left leg more marked proximally with extensor plantar response. Beside left-sided painful dysesthesias at the level of Th 8 and a dissociated sensory loss below this level contralaterally there was a depressed vibration sense as well as diminished deep tendon reflexes in both lower limbs. Routine laboratory investigations showed increased serum levels of urea and creatinine as well as moderate proteinuria suggesting diabetic nephropathy but were otherwise normal. TPHA, VDRL, autoantibodies associated with inflammatory vascular diseases, extended coagulation screening and vitamin B12 determinations were within normal ranges. Examination of the lumbar cerebrospinal fluid (CSF), including immunological studies and a search for oligoclonal bands, revealed no abnormalities.
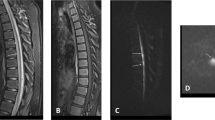
MRI was first performed on the day of hospital admission (day 1). Turbo spin echo (TSE) T1- and T2-weighted images in sagittal planes before and after intravenous administration of gadolinium failed to demonstrate alterations of signal or contour of the spinal cord. Moreover, there were no abnormal bone marrow signals in the vertebral column (Figure 1). The X-ray film of the thoracic spine as well as the skeletal scintigram of the vertebral column were unobtrusive. The X-ray film of the chest showed sclerosis of the aorta. Abdominal sonography revealed severe sclerosis of the abdominal aorta but no aneurysm or dissection. Transthoracal echocardiography failed to demonstrate intracavitary thrombi or a cardiac embolic source. Motor evoked potentials (MEP) of both anterior tibial muscles after cortical and spinal magnetic stimulation were performed on January 4, 2000 (day 13) and showed normal amplitudes and latencies. The central conduction time was normal bilaterally. Nerve conduction studies and electromyography showed signs of a mild sensorimotor mixed type neuropathy most likely due to diabetes mellitus. On January 12, 2000, (day 21) no signs of acute denervation could be detected on electromyographic examination of the left-sided paraspinal thoracic musculature.
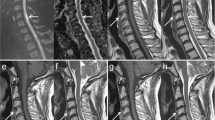
Follow-up MRI of the thoracolumbar region was performed on January 10, 2000, (day 19). TSE T1- and T2-weighted images before and after administration of gadolinium showed no signal alterations within the spinal cord but revealed a hyperintense signal in the dorsal part of the vertebra Th 9 on T2-weighted images (Figure 2). On January 13, 2000, (day 22) MRI of the thoracolumbar region with spectral fat suppression showed an improved demarcation of the abnormal signal in vertebra Th 9 and additionally an equivalent signal alteration in the dorsal part of Th 10 (Figure 3) consistent with vertebral body infarction while no signs of spinal cord ischemic stroke were detectable.
MRI (1,5 T) of the thoracolumbar region on day 22 with spectral fat suppression. (a) Left parasagittal: abnormally high signal in the dorsal area of vertebral bodies Th 9 and Th 10 attributable to high water content and bone marrow edema (TSE; TR, 4000 ms; TE, 108 ms). (b) Axial: pronounced demarcation of the abnormal signal in vertebra Th 9 corresponding to vertebral body infarction (TSE; TR, 4600 ms, TE, 180 ms)
The patient was treated with subcutaneous heparin and clopidogrel beside intensive physiotherapy. Over a period of 4 weeks he improved continuously and was transferred to a rehabilitation center on January 18, 2000 (day 27). At that time he presented with a discrete paresis of the left leg accentuated proximally. Plantar response was flexor bilaterally. Moreover, the left-sided painful dysesthesias at the level of Th 8 showed complete remission while there was a persistent dissociated sensory disturbance below this level on the right side.
Discussion
Besides signs of diabetic neuropathy this patient presented with an acute spontaneous spinal cord syndrome suggesting ischemic stroke in the territory of the left sulcocommissural artery at the level of Th 8. The left and right sulcocommissural arteries are the penetrating extensions of the anterior spinal artery supplying the grey and inner white matter. The vascular nature of this event was supported by a history of multiple vascular risk factors and coronary heart disease, normal CSF findings and the exclusion of a compressive myelopathy by MRI. However, a definite etiological diagnosis could not be made at the time of onset of symptoms.
Over the last decade several reports described abnormal bone marrow signals in the vertebral column revealed by serial MRI investigations accompanying spinal cord ischemic stroke.6 Since there are difficulties to conceive of a single pathological process other than a vascular event that can affect acutely both the spinal cord and the spinal column without affecting the intervening epidural and subarachnoid spaces these bone marrow hyperintensities on T2-weighted images were interpreted as vertebral body infarctions.7
Follow-up MRI investigations in our patient revealed vertebral body infarction of Th 9 and Th 10 in their dorsal parts. In contrast to the spinal cord territory the vertebral column receives its major blood supply from the paired segmental arteries or their equivalents at the same and/or the adjacent level. In the midthoracic region the dorsal part of a vertebral body is supplied by the posterior central artery deriving from the paired spinal branches at the level of the intervertebral foramen. The posterior central arteries further divide into cranial and caudal branches supplying two adjacent vertebral bodies.4 Therefore, occlusion of the left posterior central artery between Th 9 and Th 10 may cause infarction of vertebra Th 9 and Th 10 in their left and dorsal parts. The anterior medullary arteries, originating inconstantly and unilaterally from the spinal branches of the paired segmental arteries contribute blood to the anterior spinal artery and so to the sulcocomissural vessels in a variable number of 6 to 10.8 The segment Th 8 of the spinal cord, which was clinically affected in our patient, is located at a higher level in the transversal plane than vertebra Th 9 and 10. This difference in level of ischemia can be considered as a consequence of the ascending course of the medullary artery following the nerve roots with an increasing obliquity from cranial to caudal levels.8
In summary, the combined infarction of vertebral bodies and spinal cord in our patient was most likely due to a fragmented embolus deriving from aortic plaques. Vertebral body hyperintensities on T2-weighted MRI are not specific for infarction. They only indicate an increased water content of the osseous tissue which may also occur in fractures or metastases of vertebral bodies as well as in spondylitis. These conditions, however, should present with abnormalities of the spinal skeletal scintigram which was inconspicuous in our patient. On the other hand MRI is the only method which may reveal vertebral body infarction.9
Sequential MRI failed to demonstrate signal abnormalities within the spinal cord proving infarction in our patient. The vertical dimensions of spinal cord infarction may range from one to 15 segments, depending on the extent of vascular occlusion and collateralization. Emboli affecting small end arteries frequently cause single segmental infarctions. We assumed embolization as the underlying pathophysiological mechanism in our case. With respect to the clinical picture and course of the disease unilateral single segmental infarction may have resulted in only a small ischemic lesion and could account for the lack of a corresponding spinal cord signal. This hypothesis is supported by bilaterally normal MEP findings in our patient. MEP after cortical stimulation frequently showed a marked reduction of amplitudes at the anterior tibial muscles with normal latencies in patients with infarction in the anterior spinal artery territory.10 On the other hand the discordance of spinal cord and vertebral body signal change may have been a result of MRI investigation timing. One could speculate that the follow-up MRI may have been negative due to some fogging effect of an ischemic lesion.
What remains to be established in future studies is the time course of vertebral body hyperintensities in MRI associated with vascular spinal cord syndromes. Moreover, the time course of spinal cord MRI changes due to infarction needs further clarification. Recent single case reports support a possible diagnostic impact of diffusion-weighted MR imaging (DWI) in acute and subacute anterior spinal artery infarction.11,12 Compared to investigations of the brain, however, spinal DWI is technically more demanding owing to the small size of the spinal cord and motion-induced artifacts. To date, there are many technical and physiologic problems to overcome before DWI of the spinal cord becomes an accepted and routinely used protocol.
Our case is, to the best of our knowledge, the first report on vertebral body infarction as the only confirmatory sign of spontaneous spinal cord infarction in the territory of the anterior spinal artery. Faig et al6 reported on a patient with posterior spinal artery syndrome below Th 11. A follow-up MRI on day 14 showed a T2 hyperintense signal in the dorsal area of vertebral body Th 10 beside an unremarkable signaling of the spinal cord in the thoracic region. Since this patient had a calcified disk protrusion or small meningeoma in the vicinity of the infarcted vertebra, the most likely mechanism of spinal cord infarction was fibrocartilagenous embolism, a condition that has never been diagnosed on clinical grounds.3
We conclude that in clinically presumed spinal cord infarction follow-up MRI investigations may fail to demonstrate signal alterations within the spinal cord. In those cases vertebral body infarction may serve as the only confirmatory sign of spinal cord ischemic stroke and must be taken into consideration during sequential MRI.
References
Sandson TA, Friedman JH . Spinal cord infarction: report of 8 cases and review of the literature Medicine 1989 68: 282–292
Cheshire WP, Santos CC, Massey EW, Howard Jr JF . Spinal cord infarction: etiology and outcome Neurology 1996 47: 321–330
Mikulis DJ et al. Spinal cord infarction and fibrocartilagenous emboli AJNR Am J Neuroradiol 1992 13: 155–160
Yuh WTC et al. MR imaging of spinal cord and vertebral body infarction AJNR Am J Neuroradiol 1992 13: 145–154
Haddad MC, Aabed Al-Thagafi MY, Djurberg H . MRI of spinal cord and vertebral body infarction in the anterior spinal artery syndrome Neuroradiology 1996 38: 161–162
Faig J, Busse O, Salbeck R . Vertebral body infarction as a confirmatory sign of spinal cord ischemic stroke Stroke 1998 29: 239–243
Case records of the Massachusetts General Hospital. Case 5-1991 N Engl J Med 1991 324: 322–332
Sliwa JA, Maclean IC . Ischemic myelopathy: a review of spinal vasculature and related clinical syndromes Arch Phys Med Rehabil 1992 73: 365–372
Engellau L et al. Magnetic resonance imaging and MR angiography of endoluminally treated abdominal aortic aneurysms Eur J Vasc Endovasc Surg 1998 15: 212–219
Berlit P et al. Spinal cord infarction: MRI and MEP findings in three cases J Spinal Disord 1992 5: 212–216
Bammer R et al. Diffusion-weighted MR imaging of the spinal cord AJNR Am J Neuroradiol 2000 21: 587–591
Muscat P et al. Vertebral artery dissection in Turner's syndrome: diagnosis by magnetic resonance imaging J Neuroimaging 2001 11: 50–54
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Börnke, C., Schmid, G., Szymanski, S. et al. Vertebral body infarction indicating midthoracic spinal stroke. Spinal Cord 40, 244–247 (2002). https://doi.org/10.1038/sj.sc.3101285
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.sc.3101285
Keywords
This article is cited by
-
Vertebral body infarction revealed by diffusion-weighted magnetic resonance imaging
Journal of Neurology (2013)
-
Concomitant spinal cord and vertebral body infarction is highly associated with aortic pathology: a clinical and magnetic resonance imaging study
Journal of Neurology (2009)
-
Fibrokartilaginäre Embolie des Rückenmarks
Der Nervenarzt (2005)