Abstract
Design: Single case report.
Objective: To report a rare case of cytomegalovirus (CMV)-associated transverse myelitis (TM) in the immunocompetent host.
Settings: Collaboration between a Neurology and Radiology University Department in Greece and a Molecular Virology Department in Cyprus.
Patient: A 16-year-old male student developed an acute febrile illness followed shortly by TM, that resulted in paraplegia over 24 h. Rapid clinical improvement was followed by complete recovery in 2 months. Extensive laboratory work-up excluded other possible causes of TM and showed no evidence of an immunocompromised state. Antiviral serological data, identification of the viral genome by polymerase chain reaction and serial spinal cord magnetic resonance imaging findings, supported the diagnosis of CMV-associated TM in a non-immunocompromised patient.
Conclusions: Our case further indicates that CMV infection should be included in the differential diagnosis of TM of uncertain etiology, in the immunocompetent patient. Clinical, immunological and neuroimaging findings indicate that post-infectious immune mediated inflammation, seems the most probable pathogenetic mechanism in this case.
Similar content being viewed by others
Introduction
Cytomegalovirus (CMV)-associated transverse myelitis (TM) is rare in the immunocompetent host. We have only been able to find six cases described in the literature and in these the clinical presentation was not accompanied by concomitant immunological and neuro-imaging findings.
We report a further rare case including immunological and neuroimaging results.
Case Report
A 16-year-old, previously healthy male student was admitted on December 10, 1999, for evaluation of paraplegia that had rapidly developed over 24 h. One week earlier, he had an illness characterized by fever (38°C), sore throat and malaise that partially resolved in 48 h. This temporary improvement was followed by febrile relapse (39°C), myalgias and bilateral foot numbness 2 days before admission. Twenty-four hours later he experienced bilateral leg weakness of rapidly increasing severity.
General physical examination was unremarkable except for pharyngitis, mild cervical adenopathy and body temperature of 37.5°C. Neurological examination showed clear sensorium and normal cranial nerves, upper extremity strength, sensation and deep tendon reflexes. There was complete paralysis of both legs with increased tendon reflexes and extensor plantar responses.
Abdominal skin reflexes were absent, pain and light touch sensation were reduced below the fourth thoracic level bilaterally, while vibration and joint position sense was normal. Urinary retention and bowel incontinence were present and a Foley catheter drained 2 liters of urine.
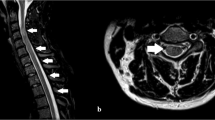
White blood cell count was 10.9×109/L with 0.48 polymorphonuclear leukocytes, 0.34 lymphocytes, 0.16 monocytes and 0.02 band forms. The hematocrit was 0.38, hemoglobin 13 g/dL, platelet count 202×109/L and erythrocyte sedimentation rate 40 mm/h. The heterophil antibody test was negative, serum coagulation, lipid, rheumatic and collagen disease studies and routine chemistry tests were normal except for serum glutamic oxalacetic transaminase (SGOT) 134 U/L (normal range 0–37 U/L), glutamic pyruvic transaminase (SGPT) 56 U/L (normal 0–40), alkaline phosphatase (AP) 133 U/L (normal range 39–117) and lactate dehydrogenase (LDH) 627 U/L (normal 240–480). Serum protein electrophoresis was normal and bacterial and fungal cultures of blood, cerebrospinal fluid (CSF), throat and stool samples were negative. Serologic tests for venereal disease, tuberculosis, mycoplasma, Toxoplasma gondii, Borrelia burgdorferi, Campylobacter, HIV, HTLV-1, hepatitis A, B and C viruses were also negative. CSF examination provided no cells, glucose 59 mg%, proteins 15 mg% and no oligoclonal bands. Nerve conduction velocity studies and f-waves as well as a brain and lumbar spine magnetic resonance imaging (MRI) (Philips Gyroscan, S15-ACS, 1.5T) with contrast were normal. Sagittal T2-weighted images (wi) of the cervical (C) – thoracic (TH) spine, demonstrated an oblong intramedullary increased signal, extending from C6 to TH9 spinal level (Figure 1a,b). Axial T2-wi at TH3 level verified the intraspinal signal location (Figure 1c). T1-wi were normal and gadolinium infusion was unrevealing. Thirteen days later, repeat spinal MRI scan revealed normal C cord and axial T2-wi showed significant improvement at the TH level (Figure 1d). At the same time bowel function and the serum levels of SGOT, SGPT, AP and LDH returned to normal. Initially, the patient was treated with intravenous (IV) infusions of Acyclovir 5 mg/kg for 5 days, along with IV human immunoglobulin (IVIg) infusions 0.4 g/kg (December 11 to 16, 1999), followed by a 5-day course of methylprednisolone IV infusions 1 g/d and intensive physiotherapy. One week after admission, he started a slow, steady clinical improvement in the motor function of both legs, first observed in the distal and later in proximal muscles. Neurological examination at discharge, demonstrated pyramidal tract signs with clonus at both lower limbs, spastic gait with canes and a mild sensory level at TH9. At 2 months, further clinical improvement was evidenced by his ability to walk independently, return of bladder function to normal and was associated with complete resolution of the high intraspinal signal in a third MRI study.
Spinal cord neuroimaging findings. Sagittal T2-weighted magnetic resonance images (repetition time 4000 ms, echo time 90 ms) demonstrate increased signal extending from the lower cervical (a) to the thoracic (b) spinal cord (arrows). Axial T2-weighted image (c) verifying the intraspinal signal location (arrow). Thirteen days later, significant improvement is noted at the same level (d) (repetition time 2000 ms, echo time 150 ms)
Virological serology
On December 21 and 29, 1999, January 3 and 10, 2000 as well as on April 10, 2000 blood samples were obtained in order to determine the levels of serum IgG and IgM antibodies against the following viruses, using commercially available enzyme-linked immunosorbent assay (ELISA). Cytomegalovirus (CMV)-antibodies (Gull, Meridian Diagnostics, Inc, USA), Herpes simplex virus-1 and 2 (HSV-1 and 2) (r-Biopharm GmbH, USA), Varicella zoster virus (VZV) (Human Gesellschaft für Biochemica und Diagnostica GmbH, Germany), Epstein-Barr virus (EBV) IgG antibodies to early antigen (EA), nuclear antigen (NA) and EBV-IgM antibodies (Sanofi-Diagnostics, Pasteur, France) and finally Enteroviruses IgG and IgM antibodies (against the three serotypes of poliovirus, 23 serotypes of Coxsackievirus A, six serotypes of Coxsackievirus B, 32 serotypes of Echovirus and the serotypes 68–71 of Enterovirus) (Genzyme Virotech GmbH, Germany) were detected as recommended by the manufacturers. The results of these antiviral serological assays are presented in Table 1. In contrast to all other viruses tested, there was a stepwise increase of CMV-IgG titer, showing a significant rise at the end, along with parallel impressive decrease of CMV-IgM antibodies, reaching negative value, after initial increase (Table 1). These results for CMV were confirmed using different reagents (Microparticle Enzyme Immunoassay, MEIA, Abbott Diagnostics, USA). Serum IgG and IgM antibodies against HSV-1, VZV, EBV and Enteroviruses revealed steadily decreasing titers, over the same time period, despite initial multiple positivity. Titers of HSV-2 were low and negative throughout (Table 1).
Viral-DNA detection
The Polymerase chain reaction (PCR) method combined with Southern blot hybridization1 was used for the detection of viral genomic material of CMV, HSV-1, HSV-2, VZV, EBV and Enteroviruses in serum samples, peripheral blood lymphocytes (PBLs) and CSF on December 21, 1999 and again only in the serum for CMV and EBV on April 10, 2000. The primers used for CMV amplification were from the fourth exon of the HCMV-IEA-1 gene located in the EcoRI fragment of strain Ad169. The amplification was performed by nested PCR as already described.2 For EBV amplification, two different pairs of primers were used, one in the Bam W region of the EBV genome and the other in the Bam M region. These two regions are reiterated 10 times in the EBV genome and the PCR was performed as described previously.3 The results of viral-DNA detection are summarized in Table 2. CMV-DNA sequences were detected both in the serum and PBLs, whereas EBV-DNA was only positive in PBLs on December 21, 1999. No viral-DNA was detected in the CSF. PCR for HSV-1, HSV-2, VZV and Enteroviruses-DNA was negative in all specimens tested. Neither CMV or EBV-DNA was finally detected in the serum on April 10, 2000 (Table 2).
Discussion
In a recent review,4 transverse myelitis (TM) was attributed to parainfectious states in 46% of cases and many viruses have been reported as causative.5 CMV-associated TM is rare in the immunocompetent host. Until 1993 only four cases had been described.5,6,7,8 However, they all lacked MRI findings and the recently developed PCR method for viral-DNA detection.2,9 In 1995 and 1999 two additional cases were published.10,11 In the patient from Pakistan,10 spinal MRI was normal and PCR was not performed, while in the patient from Italy,11 no details or images were provided concerning the ‘hyperintense signal affecting the lower level of the thoracic spinal cord’ and the PCR for CMV-DNA was positive in the serum and negative in the CSF,11 as in our case. Nevertheless, our case compared to the above5,6,7,8,10,11 is unique for several reasons:
-
1
The acute febrile illness was similar to infectious mononucleosis8,11 and was shortly followed by the development of TM. An extensive laboratory work-up excluded other possible causes of TM and showed no evidence of an immunocompromised state. Concomittant antiviral serological data, over serial determinations, identification of the viral genome by PCR and serial spinal cord MRI findings supported the diagnosis of CMV-associated TM
-
2
The serological profile for CMV antibodies (stepwise increase of IgG that persisted after 4 months and parallel decrease of IgM, reaching negative value after initial increase) along with the CMV-DNA detection both in the serum and PBLs, evidenced a recent primary infection by CMV.11 Although without tissue biopsy, it may be difficult or impossible to distinguish between direct spinal cord viral invasion and tissue injury,5,10 CMV-induced vasculitis5 or post-infectious immune-mediated process,5,12 our patient's impressive clinical recovery and complete MRI resolution, along with the negative CMV-DNA detection in the CSF, rather support the latter pathogenetic mechanism
-
3
EBV-DNA was detected in PBLs but not in the serum or the CSF and was accompanied by increased serum titers of NA-IgG, EA-IgG and IgM antibodies that all progressively declined and were found negative (EA-IgG, IgM) or very low (NA-IgG) at 4 months. These findings were indicative of no multiplication of EBV and rather corresponded to intercurrent remnants of EBV genomic material, possibly due to a past infection, with no relevance to the current illness. Another similar report however, has presented association with an intercurrent EBV reactivation, possibly elicited by the presence of CMV primary infection.13 Nevertheless, in that case, EBV-DNA was detected in the CSF, CMV-DNA was absent from the early collected CSF and the patient presented combined TM and less severe encephalitis and polyradiculopathy.13
-
4
Initial multiple antiviral positivity observed in the serum for the other viruses tested, (HSV-1, VZV, EBV and enteroviruses), possibly reflected passive serum enhancement, when the patient received human IVIg treatment14 for 5 days. Such treatment may increase viral titers and may affect serologic tests for as long as 30 days after therapy.14
In conclusion, we believe that our case further indicates that CMV infection, should be included in the differential diagnosis of TM of uncertain etiology, even if the patient is immunocompetent. Clinical, immunological and neuroimaging findings indicate that post-infectious immune-mediated inflammation seems the most probable pathogenetic mechanism in this case.
References
Mullis K et al. Specific enzymatic amplification of DNA in vitro: the polymerase chain reaction Cold Spring Harb Symp Quant Biol 1986 51: 263–273
Brytting M et al. Cytomegalovirus DNA detection of an immediate early protein gene with nested primer oligonucleotides J Virol Methods 1991 32: 127–138
Saito I, Servenius B, Compton T, Fox RI . Detection of Epstein-Barr virus DNA by polymerase chain reaction in blood and tissue biopsies from patients with Sjogren's syndrome J Exp Med 1989 169: 2191–2198
Douglas RJ, Raul NM, Larry ED . Transverse myelitis Arch Neurol 1993 50: 532–535
Tyler KL, Gross RA, Cascino GD . Unusual viral causes of transverse myelitis: Hepatitis-A virus and cytomegalovirus Neurology 1986 36: 855–858
Chin W, Magoffin R, Frierson JG, Lennette EH . Cytomegalovirus infection: a case with meningoencephalitis JAMA 1973 225: 740–741
Kabins S, Keller R, Naragi S, Peitchel R . Viral ascending radiculomyelitis with severe hypoglycorrhachia Arch Intern Med 1976 136: 933–935
Miles C, Hoffman W, Lai CW, Freeman JW . Cytomegalovirus-associated transverse myelitis Neurology 1993 43: 2143–2145
Weber T et al. Clinical implications of nucleic acid amplification methods for the diagnosis of viral infections of the nervous system J Neurovirol 1996 2: 175–190
Baig SM, Khan MR . Cytomegalovirus-associated transverse myelitis in a non-immunocompromised patient J Neurol Sci 1995 134: 210–211
Giobbia M et al. Cytomegalovirus-associated transverse myelitis in a non-immunocompromised patient Infection 1999 27: 228–230
DeBiasi RL, Tyler KL . Polymerase chain reaction in the diagnosis and management of central nervous system infections Arch Neurol 1999 56: 1215–1219
Merelli E et al. Encephalomyeloradiculopathy associated with Epstein-Barr virus: primary infection or reactivation? Acta Neurol Scand 1997 96: 416–420
Dalakas MC . Intravenous immune globulin therapy for neurological diseases Ann Intern Med 1997 126: 721–730
Acknowledgements
The authors wish to thank Prof Raymond D Adams, MD, Boston, USA for his invaluable advice during the preparation of this manuscript. The study was supported by a Cyprus TELETHON Grant (1998–2000) allocated to the Molecular Virology Department, The Cyprus Institute of Neurology and Genetics, Nicosia, Cyprus.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Karacostas, D., Christodoulou, C., Drevelengas, A. et al. Cytomegalovirus-associated transverse myelitis in a non-immunocompromised patient. Spinal Cord 40, 145–149 (2002). https://doi.org/10.1038/sj.sc.3101265
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.sc.3101265
Keywords
This article is cited by
-
High-dose corticosteroids for acute cytomegalovirus-associated transverse myelitis in the immunocompetent patient: a case report and systematic review
Journal of NeuroVirology (2019)
-
Cytomegalovirus-associated encephalomyelitis in an immunocompetent adult: a two-stage attack of direct viral and delayed immune-mediated invasions. case report
BMC Neurology (2016)
-
Cytomegalovirus associated transverse myelitis in an immunocompetent host with DNA detection in cerebrospinal fluid; a case report
BMC Research Notes (2012)
-
Encephalomyelitis following mumps
Spinal Cord (2005)