Abstract
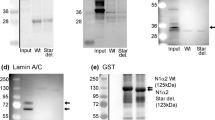
Spectrin, the major protein of the subcortical actin network in erythrocytes, contains two non-identical subunits, α and β (refs 1, 2). Spectrin is indirectly associated with the transmembrane anion transporter through the binding of β-spectrin to an extrinsic protein, ankyrin1–6. In chicken embryo erythroid cells, α-spectrin is synthesized in a threefold excess relative to β-spectrin, although the two subunits are assembled in equimolar amounts7. To investigate the regulation of assembly of equimolar amounts of spectrin, an in vitro system from chicken embryo erythroid cells has now been developed where synthesis and assembly of spectrin can be uncoupled and studied separately. Following the in vitro translation of threefold more α- than β-spectrin, 95% of the β-spectrin and equimolar amounts of α-spectrin bind post-translationally to spectrin-depleted rabbit red blood cell membranes, and the excess α-spectrin remains unbound. This α-spectrin cannot bind spectrin-depleted plasma membranes subsequently added to the lysate. The assembly of α-spectrin is, therefore, limited by the availability of β-spectrin, and both subunits assemble post-translationally.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Branton, D., Cohen, C. M. & Tyler, J. Cell 24, 24–32 (1981).
Marchesi, V. T. Blood 61, 1–11 (1983).
Bennett, V. & Stenbuck, P. J. J. biol. Chem. 254, 2532–2542 (1979).
Luna, E. J., Kidd, G. H. & Branton, D. J. biol. Chem. 254, 2526–2532 (1979).
Litman, D., Hsu, C. J. & Marchesi, B. T. J. Cell Sci. 42, 1–22 (1980).
Bennett, V. & Stenbuck, P. J. J. biol. Chem. 255, 6424–6432 (1980).
Blikstad, I., Nelson, W. J., Moon, R. T. & Lazarides, E. Cell 32, 1081–1091 (1983).
Repasky, E. A., Granger, B. L. & Lazarides, E. Cell 29, 821–833 (1982).
Nelson, W. J. & Lazarides, E. Proc. natn. Acad. Sci. U.S.A. 80, 363–367 (1983).
Granger, B. L., Repasky, E. A. & Lazarides, E. J. Cell Biol. 92, 299–312 (1982).
Bennett, V. & Branton, D. J. biol. Chem. 252, 2753–2763 (1977).
Burridge, K., Kelly, T. & Mangeat, P. J. Cell Biol. 95, 478–486 (1982).
Goodman, S. R., Zagon, I. S. & Kulikowski, R. R. Proc. natn. Acad. Sci. U.S.A. 78, 7570–7574 (1981).
Bennett, V., Davis, J. & Fowler, W. E. Nature 299, 126–131 (1982).
Lazarides, E. & Nelson, W. J. Cell 31, 505–508 (1982).
Pelham, H. R. B. & Jackson, R. J. Eur. J. Biochem. 67, 247–256 (1976).
Bradford, M. Analyt. Biochem. 72, 248–254 (1976).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Moon, R., Lazarides, E. β-Spectrin limits α-spectrin assembly on membranes following synthesis in a chicken erythroid cell lysate. Nature 305, 62–65 (1983). https://doi.org/10.1038/305062a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/305062a0
This article is cited by
-
Intertwined αβ Spectrin Meeting Helical Actin Protofilament in the Erythrocyte Membrane Skeleton: Wrap-Around vs. Point-Attachment
Annals of Biomedical Engineering (2011)
-
Silence speaks in spectrin
Nature (1994)
-
Assignment of mouse beta-spectrin gene to chromosome 12
Somatic Cell and Molecular Genetics (1987)
-
Spectrin assembly in avian erythroid development is determined by competing reactions of subunit homo- and hetero-oligomerization
Nature (1986)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.