Abstract
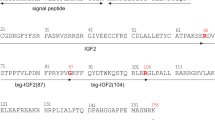
Glucagon is a 29-amino acid polypeptide hormone synthesized by the A cells of the endocrine pancreas1–4. Its primary site of action is the liver where it stimulates glycogenolysis, gluconeogenesis and ketogenesis. In mammals, biosynthetic studies have shown that glucagon is derived from a precursor of molecular weight (Mr) approximately 18,000 which is five to six times larger than glucagon5. Glucagon-containing polypeptides and immunoreactants of various sizes have also been described from stomach, intestine, brain and salivary gland3. Here, we have determined the structure of hamster pancreatic preproglucagon from the sequence of its cDNA. This 180-amino acid precursor contains the sequence of glucagon and two glucagon-like polypeptides arranged in tandem. The precursor also contains the sequences of several non-pancreatic glucagon-containing polypeptides which suggests that, in mammals, both pancreatic and non-pancreatic glucagon and glucagon-containing polypeptides may be derived from a common precursor by tissue-specific processing. We have tentatively identified each of the glucagon-like immunoreactants which have been described with respect to the sequence of proglucagon and have proposed a scheme for the processing of pancreatic proglucagon.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Foa, P., Bajaj, J. & Foa, N. (eds) Glucagon: Its Role in Physiology and Clinical Medicine (Springer, Berlin, 1977).
Unger, R.H. & Orei, L. (eds) Glucagon. Physiology, Pathophysiology and Morphology of the Pancreatic A-Cell (Elsevier, New York, 1981).
Conlon, J. M. Diabetologia 18, 85–88 (1980).
Unger, R. H. & Orci, L. New Engl J. Med. 304, 1518–1524 (1981).
Patzelt, C., Tager, H. S., Carroll, R. S. & Steiner, D. F. Nature 282, 260–266 (1979).
Proudfoot, N. J. & Brownlee, G. G. Nature 263, 211–214 (1976).
Fitzgerald, M. & Shenk, T. Cell 24, 251–260 (1981).
Carmichael, G. C. & McMaster, G. K. Meth. Enzym. 65, 380–391 (1980).
Thomas, P. S. Proc. natn. Acad. sci. U.S.A. 77, 5201–5205 (1980).
Thim, L. & Moody, A. J. Regulatory Peptides 2, 139–150 (1981).
Moody, A. J., Hoist, J. J., Thim, L. & Lindkaer Jenson, S. Nature 289, 514–516 (1981).
Tager, H. S. & Steiner, D. F. Proc. natn. Acad. sci. U.S.A. 70, 2321–2325 (1973).
Bataille, D. et al. FEBS Lett. 146, 79–86 (1982).
Lund, P. K., Goodman, R. H., Dee, P. C. & Habener, J. F. Proc. natn. Acad. sci. U.S.A. 79, 345–349 (1982).
Shen, L. P., Pictet, R. L. & Rutter, W.J. Proc. natn. Acad. sci. U.S.A. 79, 4575–4579 (1982).
Chan, S. J. et al. J. biol. Chem. 256, 7595–7602 (1981).
Lund, P. K., Goodman, R.H. & Habener, J.F. J. biol. Chem. 256, 6515–6518 (1981).
Shields, D., Warren, T. G., Roth, S. E. & Brenner, M. J. Nature 289, 511–514 (1981).
Steiner, D. F., Quinn, P. S., Chan, S. J., Marsh, J. & Tager, H.S. Ann. N.Y. Acad. sci. 343, 1–16 (1980).
Tager, H. S. & Markese, J. J. biol. Chem. 254, 2229–2233 (1979).
Kirkegaard, P. et al. Nature 297, 156–157 (1982).
Lerner, R. A. Nature 299, 592–596 (1982).
Chirgwin, J. M., Przybyla, A. E., MacDonald, R. J. & Rutter, W. J. Biochemistry 18, 5294–5299 (1979).
Santerre, R. F. et al. Proc. natn. Acad. sci. U.S.A. 78, 4339–4343 (1981).
Land, H., Grez, M., Hauser, H., Lindenmaier, W. & Schutz, G. Nucleic Acids Res. 9, 2251–2266 (1981).
Villa-Komaroff, L. et al. Proc. natn. Acad. sci. U.S.A. 75, 3727–3731 (1978).
Gergen, J. P., Stern, R. H. & Wensink, P. C. Nucleic Acids Res. 7, 2115–2136 (1979).
Comb, M., Seeburg, P. H., Adelman, J., Eiden, L. & Herbert, E. Nature 295, 663–666 (1982).
Beaucage, S. L. & Caruthers, M. H. Tetrahedron Lett. 22, 1859–1862 (1981).
Bell, G. I. et al. Nature 282, 525–527 (1979).
Maxam, A. M. & Gilbert, W. Meth. Enzym. 65, 499–560 (1980).
Dayhoff, M. O. Atlas of Protein Sequence and Structure Vol. 5, Suppl. 2, 125–126 (National Biomedical Research Foundation, Washington, DC, 1976).
Tatemoto, K. & Mutt, V. Proc. natn. Acad. sci. U.S.A. 78, 6603–6607 (1981).
Jornval, H. et al. FEBS Lett. 123, 205–216 (1981).
Guillemin, R. et al. Science 218, 585–587 (1982).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bell, G., Santerre, R. & Mullenbach, G. Hamster preproglucagon contains the sequence of glucagon and two related peptides. Nature 302, 716–718 (1983). https://doi.org/10.1038/302716a0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/302716a0
This article is cited by
-
The expanding incretin universe: from basic biology to clinical translation
Diabetologia (2023)
-
Discovery of the GI Effects of GLP-1: An Historical Perspective
Digestive Diseases and Sciences (2022)
-
Logical design of oral glucose ingestion pattern minimizing blood glucose in humans
npj Systems Biology and Applications (2019)
-
The Long Road to the Development of Effective Therapies for the Short Gut Syndrome: A Personal Perspective
Digestive Diseases and Sciences (2019)
-
The role of pollutants in type 2 diabetes mellitus (T2DM) and their prospective impact on phytomedicinal treatment strategies
Environmental Monitoring and Assessment (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.