Abstract
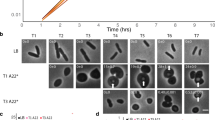
The development of convenient methodology to study penicillin-sensitive enzymes (PSEs) as penicillin-binding proteins (PBPs)1,2 has revealed their roles in bacterial cell shape determination, particularly in the Gram-negative rod Escherichia coli3–6. By analysing PBPs during the life cycle of Bacillus megaterium KM we report here the first data suggesting specific roles for these proteins in the morphogenesis of a Gram-positive rod. During the transition from the vegetative rod to the spherical/ellipsoidal-shaped dormant spore, novel PBPs are synthesized and existing ones are proteolytically modified. The differentiation of the dormant spore PBP profile back to that of the vegetative cell during germination, follows a defined sequence clearly correlated to specific changes in shape, culminating in a highly synchronous vegetative cell division.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Blumberg, P. M. & Strominger, J. L. J. biol. Chem. 247, 8107–8113 (1972).
Spratt, B. G. & Pardee, A. B. Nature 254, 516–517 (1975).
Spratt, B. G. Phil. Trans. R. Soc. B289, 273–283 (1980).
Matsuhashi, M. et al. in Beta-Lactam Antibiotics (ed. Mitsuhashi, S.) 203–223 (Springer, Berlin, 1981).
Ishino, F. & Matsuhashi, M. Biochem. biophys. Res. Commun. 101, 905–911 (1981).
Botta, G. A. & Buffa, D. Antimicrob. Agents Chemother. 19, 891–900 (1981).
Hitchins, A. D. & Slepecky, R. A. Nature 223, 804–807 (1969).
Ellar, D. J. Symp. Soc. gen. Microbiol. 28, 296–325 (1978).
Wilkinsoh, B. J., Deans, J. A. & Ellar, D. J. Biochem. J. 152, 561–569 (1975).
Mandelstam, J. Proc. R. Soc. B193, 89–106 (1976).
Ryter, A., lonesco, H. & Schaeffer, P. C. r. hebd. Séanc. Acad. Sci., Paris D252, 3675–3677 (1961).
Schaeffer, P., Ionesco, H., Ryter, A. & Balassa, G. in Colloques Internationaux du CNRS, Sciences Humaines (ed. Senez, J.) 529–544 (Gordon and Breach, New York, 1963).
Pitel, D. W. & Gilvarg, C. J. biol. Chem. 245, 6711–6717 (1970).
Tipper, D. J. & Gauthier, J. J. in Spores V (eds. Halvorson, H.O., Hanson, R. & Campbell, L. L.) 3–12 (Am. Soc. for Microbiol., Washington D. C., 1972).
Frehel, C. & Ryter, A. J. Bact. 144, 789–799 (1980).
Tipper, D. J., Pratt, I., Guinand, M., Holt, S. C. & Linnett, P. E. in Microbiology, 1977 (ed. Schlessinger, D.) 50–68 (Am. Soc. for Microbiol., Washington D. C., 1977).
Johnstone, K. & Ellar, D.J. Biochim. biophys. Acta 714, 185–191 (1982).
Hansen, J. N., Spiegelman, G. & Halvorson, H. O. Science 168, 1291–1298 (1970).
Bulla, L. A. et al. CRC Crit. Rev. Microbiol. 8, 147–204 (1980).
Chase, H. A., Shepherd, S. T. & Reynolds, P. E. FEBS Lett. 76, 199–203 (1977).
Fordham, W. D. & Gilvarg, C. J. biol. Chem. 249, 2478–2482 (1974).
Markiewicz, Z., Broome-Smith, J. K., Schwarz, U. & Spratt, B. G. Nature 297, 702–704 (1982).
Reynolds, P. E., Shepherd, S. T. & Chase, H. A. Nature 271, 568–570 (1978).
Taku, A., Stuckey, M. & Fan, D. P. J. biol. Chem. 257, 5018–5022 (1982).
Mirelman, D. in Bacterial Outer Membranes (ed. Inouye, M.) 157–166 (Wiley, New York, 1979).
Tishler, P. V. & Epstein, C. J. Analyt. Biochem. 22, 89–98 (1968).
Stewart, G. S. A. B., Johnstone, K., Hagelberg, E. & Ellar, D. J. Biochem. J. 198, 101–106 (1981).
Ellar, D. J. & Posgate, J. A. in Spore Research, 1973 (eds Barker, A. N., Gould, G. W. & Wolf, J.) 21–40 (Academic, London, 1973).
Stewart, G. S. A. B. & Ellar, D. J. Biochem. J. 202, 231–241 (1982).
Crafts-Lighty, A. & Ellar, D. J. J. appl. Bact. 48, 134–145 (1980).
Guinand, M., Vacheron, M. J., Michel, G. & Tipper, D. J. J. Bact. 138, 126–132 (1979).
Racine, F. M. & Vary, J. C. J. Bact. 143, 1208–1214 (1980).
Wilkinson, B. J., Ellar, D. J., Scott, I. R. & Koncewicz, M. A. Nature 266, 174–176 (1977).
Lowry, O. H., Rosebrough, N. J., Farr, A. L. & Randall, R. J. J. biol. Chem. 193, 265–275 (1951).
Bonner, W. L. & Laskey, R. A. Eur. J. Biochem. 46, 83–88 (1974).
Cleveland, D. W., Fischer, S. G., Kirschner, M. W. & Laemli, U. K. J. biol. Chem. 252, 1102–1106 (1977).
Chamberlain, J. P. Analyt. Biochem. 98, 132–135 (1979).
Stewart, G. & Ellar, D. J. Biochem. J. (in the press).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Todd, J., Ellar, D. Alteration in the penicillin-binding profile of Bacillus megaterium during sporulation. Nature 300, 640–643 (1982). https://doi.org/10.1038/300640a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/300640a0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.