Abstract
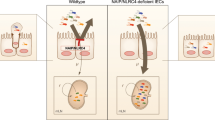
Homozygous mutations of the cystic fibrosis transmembrane conductance regulator (CFTR) cause cystic fibrosis (CF). In the heterozygous state, increased resistance to infectious diseases may maintain mutant CFTR alleles at high levels in selected populations1. Here we investigate whether typhoid fever could be one such disease. The disease is initiated when Salmonella typhi enters gastrointestinal epithelial cells for submucosal translocation2. We found that S. typhi, but not the related murine pathogen S. typhimurium, uses CFTR for entry into epithelial cells. Cells expressing wild-type CFTR internalized more S. typhi than isogenic cells expressing the most common CFTR mutation, a phenylalanine deleted at residue 508 (Δ508). Monoclonal antibodies and synthetic peptides containing a sequence corresponding to the first predicted extracellular domain of CFTR inhibited uptake of S. typhi. Heterozygous ΔF508 Cftr mice translocated 86% fewer S. typhi into the gastrointestinal submucosa than wild-type Cftr mice; no translocation occurred in ΔF508 Cftr homozygous mice. The Cftr genotype had no effect on the translocation of S. typhimurium. Immunoelectron microscopy revealed that more CFTR bound to S. typhi in the submucosa of Cftr wild-type mice than in ΔF508 heterozygous mice. We conclude that diminished levels of CFTR in heterozygotes may decrease susceptibility to typhoid fever.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Bertranpetit, J. & Calafell, F. in Variation in the Human Genome(eds Chadwick, D. & Cardew, G.) 97–114 (Wiley, Chichester, 1996).
Jones, B. D. & Falkow, S. Salmonellosis: host immune responses and bacterial virulence determinants. Annu. Rev. Immunol. 14, 533–561 (1996).
Mills, S. D. & Finlay, B. B. Comparison of Salmonella typhi and Salmonella typhimurium invasion, intracellular growth and localization in cultured human epithelial cells. Microbiol. Path. 17, 409–423 (1994).
Olsen, J. C.et al. Correction of the apical membrane chloride permeability defect in polarized cystic fibrosis airway epithelia following retroviral-mediated gene transfer. Hum. Gene Ther. 3, 253–266 (1992).
Denning, G. M.et al. Processing of mutant cystic fibrosis transmembrane conductance regulator is temperature-sensitive. Nature 358, 761–764 (1992).
Pier, G. B.et al. Role of mutant CFTR in hypersusceptibility of cystic fibrosis patients to lung infections. Science 271, 64–67 (1996).
Cheng, S. H.et al. Functional activation of the cystic fibrosis trafficking mutant ΔF508-CFTR by overexpression. Am. J. Physiol.-Lung Cell. Mol. Physiol. 12, L615–L624 (1995).
Pier, G. B., Grout, M. & Zaidi, T. S. Cystic fibrosis transmembrane conductance regulator is an epithelial cell receptor for clearance of Pseudomonas aeruginosa from the lung. Proc. Natl Acad. Sci. USA 94, 12088–12093 (1997).
Walker, J., Watson, J., Holmes, C., Edelman, A. & Banting, G. Production and characterisation of monoclonal and polyclonal antibodies to different regions of the cystic fibrosis transmembrane conductance regulator (CFTR): detection of immunologically related proteins. J. Cell Sci. 108, 2433–2444 (1995).
Flotte, T. R.et al. Stable in vivo expression of the cystic fibrosis transmembrane conductance regulator with an adeno-associated virus vector. Proc. Natl Acad. Sci. USA 90, 10613–10617 (1993).
Alpuche-Aranda, C. M., Berthiaume, E. P., Mock, B., Swanson, J. A. & Miller, S. I. Spacious phagosome formation within mouse macrophages correlates with Salmonella serotype pathogenicity and host susceptibility. Infect. Immun. 63, 4456–4462 (1995).
Ishibashi, Y. & Arai, T. Apossible mechanism for host-specific pathogenesis of Salmonella serovars. Microbiol. Path. 21, 435–446 (1996).
Pascopella, L.et al. Host restriction phenotypes of Salmonella typhi and Salmonella gallinarum. Infect. Immun. 63, 4329–4335 (1995).
Jones, B. D., Ghori, N. & Falkow, S. Salmonella typhimurium initiates murine infection by penetrating and destroying the specialized epithelial M cells of the Peyer's patches. J. Exp. Med. 180, 15–23 (1994).
Colledge, W. H.et al. Generation and characterization of a ΔF508 cystic fibrosis mouse model. Nature Genet. 10, 445–452 (1995).
Tsui, L. C. The cystic fibrosis transmembrane conductance regulator gene. Am. J. Respir. Crit. Care Med. 151, S47–S53 (1995).
Romeo, G., Devoto, M. & Galietta, L. J. V. Why is the cystic fibrosis gene so frequent? Human Genet. 84, 1–5 (1989).
Gabriel, S. E., Brigman, K. N., Koller, B. H., Boucher, R. C. & Stutts, M. J. Cystic fibrosis heterozygote resistance to cholera toxin in the cystic fibrosis mouse model. Science 266, 107–109 (1994).
Cuthbert, A. W., Halstead, J., Ratcliff, R., Colledge, W. H. & Evans, M. J. The genetic advantage hypothesis in cystic fibrosis heterozygotes: a murine study. J. Physiol. (Lond) 482, 449–454 (1995).
Pollitzer, R. Cholera(World Health Organization, Geneva, 1959).
Van Heyningen, W. E. & Seal, J. R. Cholera: The American Scientific Encounter, 1947–1980. 1–343 (Westview, Boulder, Colorado, 1983).
Fleiszig, S. M. J., Zaidi, T. S. & Pier, G. B. Psuedomonas aeruginosa invasion of and multiplication within corneal epithelial cells in vitro. Infect. Immun. 63, 4072–4077 (1995).
Beatty, W. L. & Sansonetti, P. J. Role of lipopolysaccharide in signaling to subepithelial polymorphonuclear leukocytes. Infect. Immun. 65, 4395–4404 (1997).
O'Riordan, C. R.et al. Purification and characterization of recombinant cystic fibrosis transmembrane conductance regulator from Chinese hamster ovary and insect cells. J. Biol. Chem. 270, 17033–17043 (1995).
Rosner, B. in Fundamentals of Biostatistics 498–503 (Duxbury, Boston, Massachusetts, 1990).
Acknowledgements
We thank J. Olsen, J. Yankaskas and L. Johnson for CFT1 cells, A. Smith and colleagues (Genzyme) for C127 cell lines, P. Zeitlin for IB3 and C38 cells, C. Lee for advice on Salmonella uptake assays, and C. Lee and D. Kasper for reading the manuscript. This work was supported by the NIH, the Cystic Fibrosis Foundation and the Cystic Fibrosis Trust.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pier, G., Grout, M., Zaidi, T. et al. Salmonella typhi uses CFTR to enter intestinal epithelial cells. Nature 393, 79–82 (1998). https://doi.org/10.1038/30006
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/30006
This article is cited by
-
Genetic defects in human azoospermia
Basic and Clinical Andrology (2019)
-
Establishment and equilibrium levels of deleterious mutations in large populations
Scientific Reports (2019)
-
Do GWAS and studies of heterozygotes for NPC1 and/or NPC2 explain why NPC disease cases are so rare?
Journal of Applied Genetics (2018)
-
Cystic fibrosis carriership and tuberculosis: hints toward an evolutionary selective advantage based on data from the Brazilian territory
BMC Infectious Diseases (2017)
-
Three-dimensional organotypic co-culture model of intestinal epithelial cells and macrophages to study Salmonella enterica colonization patterns
npj Microgravity (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.