Abstract
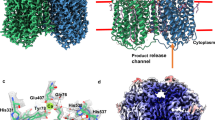
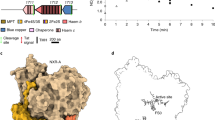
Catalase (H2O2 : H2O2-oxidoreductase, EC 1.11.1.6) is an enzyme that catalyses decomposition of hydrogen peroxide to oxygen and water, and is present in all aerobic cells. All catalases studied so far are tetrameric, each subunit (molecular weight ∼60,000) being formed by a single polypeptide chain with haemin as a prosthetic group1,2. Catalase is one of the most efficient enzymes known, resulting in reaction rates approaching the diffusion-controlled limit. Knowledge of the three-dimensional structure of catalase should help to increase our understanding of its mechanism of action, which is at present rather poor. It will also be of interest to compare the structure of catalase with that of other haem proteins, particularly from the point of view of evolutionary relationships. Thus, we have now analysed the three-dimensional structure of catalase from the fungus Penicillium vitale. From an electron density map at 3.5 Å resolution it was possible to trace the polypeptide chain. The haem group has been identified, and the active site localized using the specific inhibitor 3-amino-1:2:4-triazole. P. vitale catalase subunits have been shown to be composed of three domains, with α + β, α and α/β type secondary structures respectively. Comparison with the structure of beef liver catalase3 shows that the latter enzyme lacks the C-terminal domain of the P. vitale enzyme.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Deisseroth, A. & Dounce, A. L. Physiol. Rev. 50, 319–375 (1970).
Schonbaum, G. R. & Chnce, B. in The Enzymes Vol. 13, 3rd edn, Ch. 7 (Academic, New York, 1976).
Reid, T. J. III, et al. Proc. natn. Acad. Sci, U.S.A. (submitted).
Karpukhina, S. Ya., Barynin, V. V. & Lobanova, G. M. Kristallografiya 20, 680–681 (1975).
Vainshtein, B. K. et al. Doklady Akad. Nauk SSSR 246, 220–223 (1979).
Bricogne, G. Acta crystallogr. A32, 832–847 (1976).
Schroeder, W. A., Shelton, J. R., Shelton, J. B., Robberson, B. & Appel, G. Archs Biochem. Biophys. 131, 653–655 (1969).
Levitt, M. & Chothia, C. Nature 261, 552–557 (1976).
Watenpaugh, K. D., Sieker, L. C., Jensen, L. H., Legall, J. & Dubourdien, M. Proc. natn. Acad. Sci. U.S.A. 69, 3185–3188 (1972).
Rossmann, M. G., Moras, D. & Olsen, K. W. Nature 250, 194–199 (1974).
Vainshtein, B. K., Melik-Adamyan, W. R., Barynin, V. V. & Vagin, A. A. Dokl Akad. Nauk SSSR 250, 242–246 (1980).
Kraut, J. J. molec. Bol. 35, 511–512 (1968).
Heim, W. G., Appleman, D. & Pyfrom, H. T. Science 122, 693–694 (1955).
Margoliash, E., Novogrodsky, A. & Schejter, A. Biochem. J. 74, 339–350 (1960).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Vainshtein, B., Melik-Adamyan, W., Barynin, V. et al. Three-dimensional structure of the enzyme catalase. Nature 293, 411–412 (1981). https://doi.org/10.1038/293411a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/293411a0
This article is cited by
-
X-ray Crystallography and Electron Paramagnetic Resonance Spectroscopy Reveal Active Site Rearrangement of Cold-Adapted Inorganic Pyrophosphatase
Scientific Reports (2020)
-
Increasing BOD5/COD ratio of non-biodegradable compound (reactive black 5) with ozone and catalase enzyme combination
SN Applied Sciences (2020)
-
Modulation of the mitochondrial voltage-dependent anion channel (VDAC) by hydrogen peroxide and its recovery by curcumin
European Biophysics Journal (2020)
-
Understanding renal nuclear protein accumulation: an in vitro approach to explain an in vivo phenomenon
Archives of Toxicology (2017)
-
Amyloid-beta neuroprotection mediated by a targeted antioxidant
Scientific Reports (2014)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.