Abstract
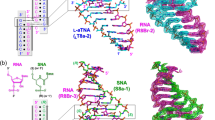
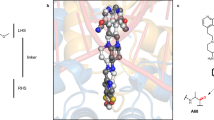
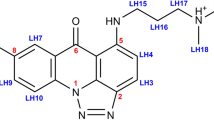
Quinoxaline antibiotics (Fig. 1a, b) form a useful group of compounds for the study of drug–nucleic acid interactions1,2. They consist of a cross-bridged cyclic octadepsipeptide, variously modified, bearing two quinoxaline chromophores. These antibiotics intercalate bifunctionally into DNA2,3 probably via the narrow groove, forming a complex in which, most probably, two base pairs are sandwiched between the chromophores4,5. Depending on the nature of their sulphur-containing cross-bridge and modifications to their amino acid side chains, they display characteristic patterns of nucleotide sequence selectivity when binding to DNAs of different base composition and to synthetic polydeoxynucleotides4,6,7. This specificity has been tentatively ascribed to specific hydrogen-bonding interactions between functional groups in the DNA and complementary moieties on the peptide ring2,4,5. Variations in selectivity have been attributed both to changes in the conformation of the peptide backbone6 and no modifications of the cross-bridge7. These suggestions were made, however, in the absence of firm knowledge about the three-dimensional structure and conformation of the antibiotic molecules. We now report the X-ray structure analysis of the synthetic analogue of the antibiotic triostin A, TANDEM (des-N-tetramethyl triostin A) (Fig. 1c), which binds preferentially to alternating adenine-thymine sequences7. The X-ray structure provides a starting point for exploring the origin of this specificity and suggests possible models for the binding of other members of the quinoxaline series.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Katagiri, K., Yoshida, T. & Sato, K. in Antibiotics Vol. 3 (eds Corcoran, J. S. & Hahn, F. E.) 234–251 (Springer, Heidelberg, 1975).
Waring, M. J. in Antibiotics Vol. 5, Pt 2 (ed. Hahn, F. E.) 173–194 (Springer, Heidelberg, 1979).
Waring, M. J. & Wakelin, L. P. G. Nature 252, 653–657 (1974).
Wakelin, L.P.G. & Waring, M. J. Biochem. J. 157, 721–740 (1976).
Cheung, H. T. et al. J. Am. chem. Soc. 100, 46–54 (1978).
Lee, J. S. & Waring, M. J. Biochem. J. 173, 115–128 (1978).
Lee, J. S. & Waring, M. J. Biochem. J. 173, 129–144 (1978).
Sheldrick, G. M. (in preparation).
Fuller, W. & Waring, M. J. Ber. Bunsenges phys. Chem. 68, 805–808 (1964).
Waring, M. J. in Drug Action at the Molecular Level (ed. Roberts, G. C. K.) 167–189 (Macmillan, London, 1977).
Ughetto, G. & Waring, M. J. Molec. Pharmac. 13, 579–584 (1977).
Wang, J. C. J. molec. Biol. 89, 783–801 (1974).
Kalman, J. R., Blake, T. J., Williams, D. H., Feeney, J. & Roberts, G. C. K. JCS Perkin I, 1313–1321 (1979).
van der Helm, D. et al. (in preparation).
Wang, A. H. J. et al. Nature 282, 680–686 (1979).
Drew, H., Takano, T., Tanaka, S., Itakura, K. & Dickerson, R. E. Nature 286, 567–573 (1980).
Levitt, M. Proc. natn. Acad. Sci. U.S.A. 75, 640–644 (1978).
Hogan, M., Dattagupta, N. & Crothers, D. M. Proc. natn. Acad. Sci. U.S.A. 75, 195–199 (1978).
Hogan, M., Dattagupta, N. & Crothers, D. M. Biochemistry 18, 280–288 (1979).
Quigley, G. J. et al. Proc. natn. Acad. Sci. U.S.A. (in the press).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Viswamitra, M., Kennard, O., Cruse, W. et al. Structure of TANDEM and its implication for bifunctional intercalation into DNA. Nature 289, 817–819 (1981). https://doi.org/10.1038/289817a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/289817a0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.