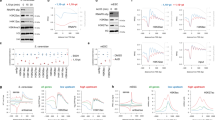
Abstract
Transcriptional co-activators were originally identified as proteins that act as intermediaries between upstream activators and the basal transcription machinery. The discovery that co-activators such as Tetrahymena and yeast Gcn51,2, as well as human p300/CBP3,4, pCAF5, Src-16, ACTR7 and TAFII2508, can acetylate histones suggests that activators may be involved in targeting acetylation activity to promoters. Several histone deacetylases have been linked to transcriptional co-repressor proteins9, suggesting that the action of both acetylases and deacetylases is important in the regulation of many genes. Here we demonstrate the binding of two native yeast histone acetyltransferase (HAT) complexes to the herpesvirus VP16 activation domain and the yeast transcriptional activator Gcn4, and show that it is their interaction with the VP16 activation domain that targets Gal4–VP16-bound nucleosomes for acetylation. We find that Gal4–VP16-driven transcription from chromatin templates is stimulated by both HAT complexes in an acetyl CoA-dependent reaction. Our results demonstrate the targeting of native HAT complexes by a transcription-activation domain to nucleosomes in order to activate transcription.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Brownell, J. E. & Allis, C. D. Special HATs for special occasions: Linking histone acetylation to chromatin assembly and gene activation. Curr. Opin. Genet. Dev. 6, 176–184 (1996).
Grant, P. A.et al. Yeast Gcn5 functions in two multisubunit complexes to acetylate nucleosomal histones: characterization of an Ada complex and the SAGA (Spt/Ada) complex. Genes Dev. 11, 1640–1650 (1997).
Ogryzko, V. V., Schiltz, R. L., Russanova, V., Howard, B. H. & Nakatani, Y. The transcriptional coactivators p300 and CBP are histone acetyltransferases. Cell 87, 953–959 (1996).
Bannister, A. J. & Kouzarides, T. The CBP coactivator is a histone acetyltransferase. Nature 384, 641–643 (1996).
Yang, X.-J., Ogryzko, V., Nishikawa, J., Howard, B. H. & Nakatani, Y. Ap300/CBP-associated factor that competes with the adenoviral oncoprotein E1A. Nature 382, 319–324 (1996).
Spencer, T. E.et al. Steroid receptor coactivator-1 is a histone acetyltransferase. Nature 389, 194–198 (1997).
Chen, H.et al. Nuclear receptor coactivator ACTR is a novel histone acetyltransferase and forms a multimeric activation complex with P/CAF and CBP/p300. Cell 90, 569–580 (1997).
Mizzen, C. A.et al. The TAFII250 subunit of TFIID has histone acetyltransferase activity. Cell 87, 1261–1270 (1996).
Struhl, K. Histone acetylation and transcriptional regulatory mechanisms. Genes Dev. 12, 599–606 (1998).
Marcus, G. A., Horiuchi, J., Silverman, N. & Guarente, L. ADA5/SPT20 links the ADA and SPT genes, which are involved in yeast transcription. Mol. Cell. Biol. 16, 3197–3205 (1996).
Roberts, S. M. & Winston, F. SPT20/ADA5 encodes a novel protein functionally related to the TAT-binding protein and important for transcription in Saccharomyces cerevisiae. Mol. Cell. Biol. 16, 3206–3213 (1996).
Kuo, M.-H., Zhou, J., Jambeck, P., Churchill, M. E. A. & Allis, C. D. Histone acetylgransferase activity of yeast Gcn5p is required for the activation of target genes in vivo. Genes Dev. 12, 627–639 (1998).
Berger, S. L.et al. Genetic isolation of ADA2: a potential transcriptional adaptor required for function of certain acidic activation domains. Cell 70, 251–265 (1992).
Piña, B.et al. ADA3: a gene, identified by resistance to GAL4-VP16, with properties similar to and different from those of ADA2. Mol. Cell. Biol. 13, 5981–5989 (1993).
Silverman, N., Agapite, J. & Guarente, L. Yeast ADA2 protein binds to the VP16 protein activation domain and activates transcription. Proc. Natl Acad. Sci. USA 91, 11665–11668 (1994).
Barlev, N. A.et al. Characterization of physical interactions of the putative transcriptional adaptor, ADA2, with acidic activation domains and TATA-binding protein. J. Biol. Chem. 270, 19337–19344 (1995).
Triezenberg, S. J., Kingsbury, R. C. & McKnight, S. L. Functional dissection of VP16, the trans-activator of herpes simplex virus immediate early gene expression. Genes Dev. 2, 718–729 (1988).
Berger, S. L., Cress, W. D., Cress, A., Triezenberg, S. J. & Guarente, L. Selective inhibition of activated but not basal transcription by the acidic activation domain of VP16: Evidence for transcriptional adaptors. Cell 61, 1199–1208 (1990).
Regier, J. L., Shen, F. & Treizenberg, S. J. Pattern of aromatic and hydrophobic amino acids critical for one of two subdomains of the VP16 transcription activator. Proc. Natl Acad. Sci. USA 90, 883–887 (1993).
Cress, W. D. & Triezenberg, S. J. Critical structural elements of the VP16 transcriptional activation domain. Science 251, 87–90 (1991).
Ingles, C. J., Shales, M., Cress, W. D., Trienzenberg, S. J. & Greenblatt, J. Reduced binding of TFIID to transcriptionally compromised mutants of VP16. Nature 351, 588–590 (1991).
Lin, Y.-S. & Green, M. G. Mechanism of action of an acidic transcriptional activator in vitro. Cell 64, 971–981 (1991).
Xiao, H.et al. Binding of basal transcription factor TFIIH to the acidic activation domains of VP16 and p53. Mol. Cell. Biol. 14, 7013–7024 (1994).
Vettese-Dadey, M., Walter, P., Chen, H., Juan, L.-J. & Workman, J. L. Role of the histone amino termini in facilitated binding of a transcription factor, GAL4-AH, to nucleosome cores. Mol. Cell. Biol. 14, 970–981 (1994).
Lin, Y. & S., Y. Carey, M. F., Ptashne, M. & Green, M. R. GAL4 derivatives function alone and synergistically with mammalian activators in vitro. Cell 54, 659–664 (1998).
Chasman, D. I., Leatherwood, J., Carey, M., Ptashne, M. & Kornberg, R. D. Activation of yeast polymerase II transcription by herpes virus VP16 and GAL4 derivatives in vitro. Mol. Cell. Biol. 9, 4746–4749 (1989).
Bradford, M. M. Arapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analyt. Biochem. 72, 248–254 (1976).
Juan, L.-J., Utley, R. T., Vignali, M., Bohm, L. & Workman, J. L. H1-mediated repression of transcription factor binding to a stably positioned nucleosome. J. Biol. Chem. 272, 3635–3640 (1997).
Steger, D. J., Eberharter, A., John, S., Grant, P. A. & Workman, J. L. Purified histone acetyltransferases stimulate HIV-1 transcription from preassembled nucleosomal arrays. Proc. Natl Acad. Sci. USA(submitted).
Côté, J., Utley, R. T. & Workman, J. L. Basic analysis of transcription factor binding to nucleosomes. Meth. Mol. Genet. 6, 108–129 (1995).
Utley, R. T.et al. In vitro analysis of transcription factor binding to nucleosomes and nucleosome disruption/displacement. Meth. Enzymol. 274, 276–291 (1996).
Acknowledgements
We thank M. Green and J. Reese for the gift of GST–VP16 and GST–VP16 mutant expression plasmids, N. Barlev and S. Berger for the GST–GCN4 expression plasmid, and members of J.L.W.'s laboratory for comments on the manuscript. This work was supported by a grant from the National Institute of General Medical Sciences. P.G. is supported by a postdoctoral fellowship from the American Cancer Society; J. C. was a recipient of an MRC Centennial fellowship; A.E. holds a fellowship from the Austrian Science Foundation; D.J.S. was supported by a postdoctoral fellowship from the Cancer Research Institute; S.J. is an HHMI postdoctoral associate and J.L.W. was a Leukemia Society scholar and is currently an HHMI investigator.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Utley, R., Ikeda, K., Grant, P. et al. Transcriptional activators direct histone acetyltransferase complexes to nucleosomes. Nature 394, 498–502 (1998). https://doi.org/10.1038/28886
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/28886
This article is cited by
-
Epigenetic modifications in the development of bronchopulmonary dysplasia: a review
Pediatric Research (2024)
-
Acetylation-dependent SAGA complex dimerization promotes nucleosome acetylation and gene transcription
Nature Structural & Molecular Biology (2022)
-
Perspectives for epigenetic editing in crops
Transgenic Research (2021)
-
The molecular logic of Nanog-induced self-renewal in mouse embryonic stem cells
Nature Communications (2019)
-
Organization and regulation of gene transcription
Nature (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.