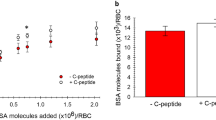
Abstract
The plasma tripeptide glycyl-L-histidyl-L-lysine (GHL), when added at nanomolar concentrations to a wide group of cultured systems, produces a disparate set of responses ranging from the stimulation of growth and differentiation to outright toxicity1–3. Such diverse actions imply that this tripeptide mediates some basic biochemical function common to many types of cells and organisms. During the isolation of GHL we found the compound to co-isolate through a number of steps with approximately equimolar copper and about 1/5 molar iron1. Maximal effects on hepatoma cells (HTC4) were seen when the peptide was added with copper and iron to the growth medium1. Structure–function studies revealed that several tripeptides with a histidyl-lysyl linkage were nearly as active as GHL1. The association of GHL with copper and a homology similarity between the tripeptide and the copper transport sites on albumin and α-fetoprotein, where the cupric atom is bound to a histidyl residue adjacent to a basic residue, suggested that GHL may act as a copper transport factor4. We report here that the tripeptide readily forms complexes with copper(II) and enhances the uptake of the metal into cultured hepatoma cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Pickart, L. & Thaler, M. Nature new Biol. 243, 85–87 (1973); J. Chromat. 175, 65–73 (1979); FEBS Lett. 104, 119–122 (1979); J. cell Physiol. 102, 129–139 (1980).
Schlesinger, D. H., Pickart, L. & Thaler, M. Experientia 33, 324–325 (1977).
Pickart, L., Thayer, L. & Thaler, M. Biochem. biophys. Res. Commun. 54, 562–566 (1973).
Aoyagi, T., Ikenaka, T. & Ichida, F. Cancer Res. 38, 3483–3486 (1978).
Peisach, J. in Mechanisms of Oxidizing Enzymes (eds Singer, T. P. & Ondarza, R. N.) 385–406 (Elsevier, Amsterdam, 1978).
Ambesi-Impiombato, F. S., Parks, L. A. M. & Coon, H. G. Proc. natn. Acad. Sci. U.S.A. 77, 3455–3459 (1980).
Robertson, J. A. J. clin. Microbiol. 7, 127–132 (1978).
Stromberg, B. E., Khoury, P. B. & Soulsby, E. J. L. Int. J. Parasit. 7, 149–151 (1977).
Dessaint, J. P., Camus, D., Fischer, E. & Capron, A. Eur. J. Immun. 7, 624–629 (1977).
Sensenbrenner, M., Jaros, G. G., Moonen, G. & Mandel, P. Neurobiology 5, 207–213 (1975).
Lindner, G., Grosse, G. & Henklein, P. Z. mikrosk.-anat. Forsch. 93, 820–828 (1979).
Simon, W. E. & Holzel, F. J. Cancer Res. clin. Oncol. 94, 307–323 (1979).
Capron, M., Capron, A., Torpier, G., Bazin, H., Bout, D. & Joseph, M. Eur. J. Immun. 8, 127–133 (1978).
Svanberg, L. & Astedt, B. Experientia 35, 818–819 (1979).
Leung, M. K., Fessier, L. J., Greenberg, D. B. & Fessler, J. H. J. biol. Chem. 254, 224–232 (1979).
Astedt, B., Barlow, G. & Holmberg, L. Thromb. Res. 11, 149–153 (1977).
Eriksson, S., Alm, R. & Astedt, B. Biochim. biophys. Acta 542, 496–505 (1978).
Joseph, M., Dessaint, J. P. & Capron, A. Cell Immun. 34, 247–258 (1977).
Capron, M., Rousseaux, J., Mazingue, C., Bazin, H. & Capron, A. J. Immun. 121, 2518–2526 (1978).
Mazingue, C., Dessaint, J. P. & Capron, A. J. immun. Meth. 21, 65–77 (1978).
Holmberg, L., Lecander, L., Persson, B. & Astedt, B. Biochim. biophys. Acta 544, 128–137 (1978).
Slotta, K. H., Golub, A. L. & Lopez, V. Hoppe-Seyler's Z. physiol. Chem. 356, 367–376 (1975).
Torpier, G., Quaissi, M. A. & Capron, A. J. ultrastruct. Res. 67, 276–287 (1979).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Pickart, L., Freedman, J., Loker, W. et al. Growth-modulating plasma tripeptide may function by facilitating copper uptake into cells. Nature 288, 715–717 (1980). https://doi.org/10.1038/288715a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/288715a0
This article is cited by
-
Structural basis for lipid and copper regulation of the ABC transporter MsbA
Nature Communications (2022)
-
Structural Characterization and Drug Delivery System of Natural Growth-Modulating Peptide Against Glioblastoma Cancer
International Journal of Peptide Research and Therapeutics (2021)
-
Microneedle-mediated delivery of cosmeceutically relevant nucleoside and peptides in human skin: challenges and strategies for dermal delivery
Journal of Pharmaceutical Investigation (2019)
-
Similarities and differences of copper and zinc cations binding to biologically relevant peptides studied by vibrational spectroscopies
JBIC Journal of Biological Inorganic Chemistry (2017)
-
Selected Biomarkers Revealed Potential Skin Toxicity Caused by Certain Copper Compounds
Scientific Reports (2016)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.