Abstract
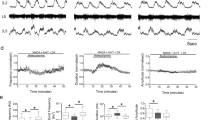
The application of serotonin to certain myenteric plexus neurones in the guinea pig small intestine causes a slow depolarization of membrane potential, accompanied by increased neuronal excitability and input resistance1. On the other hand, microiontophoretic application of large amounts of serotonin onto mammalian spinal motoneurones is reported to cause membrane hyperpolarization and decreased excitability2. However, on the basis of recording spinal reflex activity, serotonin has been reported to enhance net motoneurone activity3. Moreover, studies using extracellular single-cell recording techniques indicate that serotonin in small amounts facilitates synaptically or glutamate-induced excitation of mammalian motoneurones in the facial nucleus4 and spinal cord5. It was suggested that these facilitatory actions were modulatory in nature, as serotonin did not induce motoneurone spiking in the absence of extrinsic excitatory input. The study reported here investigated the membrane mechanisms underlying these modulatory effects by obtaining intracellular recordings from rat facial motoneurones during extracellular microiontophoretic application of serotonin, methysergide (a serotonin antagonist) and noradrenaline. Serotonin caused a slow depolarization of membrane potential of about 5 mV which remained subthreshold, accompanied by an increase in electrical excitability of the neurone, and an increase in input resistance. Noradrenaline caused the same changes. Methysergide antagonized the effects of serotonin, but not noradrenaline, indicating that these actions of serotonin are selective and receptor mediated.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Wood, J. D. & Mayer, C. J. Nature 276, 836–837 (1978); J. Neurophysiol. 42, 582–593 (1979).
Phillis, J. W., Tebecis, A. K. & York, D. H. Eur. J. Pharmac. 4, 471–475 (1968).
Barasi, S. & Roberts, M. H. T. Br. J. Pharmac. 52, 339–348 (1974).
McCall, R. B. & Aghajanian, G. K. Brain Res. 169, 11–27 (1979).
White, S. R. & Neuman, R. S. Brain Res. 188, 119–127 (1980).
Fuxe, K. Acta physiol. scand. 64, 247, 37–85 (1965).
Palkovits, M., Brownstein, M. & Saavedra, J. M. Brain Res. 80, 237–249 (1974).
Wood, J. D., Grafe, P. & Mayer, C. J. Soc. Neurosci. Abstr. 5, 749 (1979).
Gerschenfeld, H. M. & Paupardin-Tritsch, D. J. Physiol., Lond. 243, 427–456 (1974).
Woodward, D. J., Moises, H. C., Waterhouse, B. D., Hoffer, B. J. & Freedman, R. Fedn Proc. 38, 2109–2116 (1979).
Aghajanian, G. K. & McCall, R. B. Neuroscience (in the press).
Green, A. R. & Grahame-Smith, D. G. Nature 260, 487–491 (1976).
Jacobs, B. Life Sci. 19, 777–785 (1976).
Kitai, S. T., Tanaka, T., Tsukahara, N. & Yu, H. Expl Brain Res. 16, 161–183 (1972).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
VanderMaelen, C., Aghajanian, G. Intracellular studies showing modulation of facial motoneurone excitability by serotonin. Nature 287, 346–347 (1980). https://doi.org/10.1038/287346a0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/287346a0
This article is cited by
-
Reduced cholinergic and glutamatergic synaptic input to regenerated motoneurons after facial nerve repair in rats: potential implications for recovery of motor function
Brain Structure and Function (2014)
-
Hierarchy of orofacial rhythms revealed through whisking and breathing
Nature (2013)
-
Raphe Stimulation-Evoked Modulation of Postsynaptic Responses by Neurons of the Cat Somatosensory Cortex Activated by Stimulation of Nociceptors
Neurophysiology (2011)
-
Modulation of the Acoustic Startle Response by the Level of Arousal: Comparison of Clonidine and Modafinil in Healthy Volunteers
Neuropsychopharmacology (2007)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.