Abstract
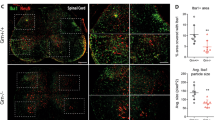
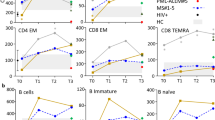
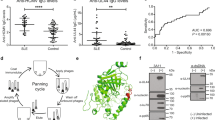
MEASLES VIRUS usually causes an acute self-limiting infection in humans and a productive cytolytic infection in susceptible tissue culture cells. However, measles-like virus has been isolated from the brains of patients with subacute sclerosing panencephalitis (SSPE), a chronic progressive neurological disorder of children1. Also, persistent measles infection has been implicated in the pathogenesis of several chronic diseases of unknown aetiology, including multiple sclerosis2 and systemic lupus erythematosus (SLE)3. Manipulation of the conditions of infection can result in persistently infected tissue culture cells, which have been proposed as a model system for studying the molecular mechanisms responsible for cytolytic virus persistence in man4–6. There is experimental evidence for at least four mechanisms leading to the induction of persistent measles infections: the production of defective virus particles which interfere with the replication of complete virus7, the generation of mutant virus strains with the capacity for non-cytolytic replication8, and infection of certain cell types (for example, neurones) restricted in their capacity to support all steps in virus replication9. Zhdanov et al.10–13 have proposed that measles virus persists in cells by a mechanism similar to that shown for retroviruses, that is, the synthesis of a DNA copy of the viral genome and the insertion of the proviral DNA into the host cell chromosome. This would be a novel mode of replication, as the replication of measles virus in cytolytic infections involves a double-stranded RNA intermediate. Using tritium-labelled viral RNA probes in molecular hybridisation assays, Zhdanov et al. have demonstrated the presence of measles virus-related DNA in chick embryo cells11 and human lymphoid cells13 persistently infected with measles virus. Further, they detected measles virus-related DNA in leukocytes, lymph nodes, kidney and urine cell sediment from five SLE patients12. Negative results were obtained using cells from appropriate control tissues. The obligative reverse transcriptase was found in the persistently infected cell lines and SLE tissues, and concomitant retrovirus infection was proposed as a prerequisite for persistent measles infection. Thus, these studies not only provide insights into the molecular mechanisms of measles virus persistence but suggest aetiological relationships for a fatal disease of unknown cause. However, we report here that, using a more sensitive nucleic acid hybridisation assay, we were unable to confirm the presence of measles virus-related DNA in either persistently infected cells or cells from patients with SLE.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Horta-Barbosa, L., Fucillo, D., Sever, J. & Zeman, W. Nature 221, 974 (1969).
Brody, J. Lancet i, 173 (1972).
Viola, M. V., Scott, C. S. & Duffy, P. Arthritis Rheum. 5, 546 (1978).
Rustigian, R. J. Bact. 92, 1792 (1966).
Norrby, E. Arch. ges. Virusforsch. 20, 216 (1967).
Knight, P., Duff, R. & Rapp, F. J. Virol. 10, 995–1001 (1972).
Huang, A. & Baltimore, D. Nature 226, 325 (1970).
Preble, R. & Younger, J. J. infect. Dis. 131, 467 (1975).
Raine, C., Feldman, L., Sheppard, R. & Bornstein, J. J. Virol. 8, 318 (1971).
Zhdanov, V. & Parfanovich, M. Arch. ges. Virusforsch. 45, 225 (1974).
Alekberova, Z., Parfanovich, M., Nassonova, V. & Zhdanov, V. Arch. Virusforsch. 47, 109 (1975).
Zhdanov, V. Nature 256, 471 (1975).
Barinskii, I. F. et al. Proc. natn. Acad. Sci. USSR 238, 236–238 (1978).
Rothenberg, E. & Baltimore, D. J. Virol. 17, 168 (1976).
Kacian, D. & Myers, J. Proc. natn. Acad. Sci. U.S.A. 73, 2191 (1976).
Hall, W. W. & ter Meulen, V. J. gen. Virol. 34, 391 (1977).
Kiley, M. & Payne, F. J. Virol. 14, 758 (1974).
Viola, M. V. & Norton, P. J. Virol. (in the press).
Studier, F. J. molec. Biol. 11, 373 (1965).
Birnstiel, M., Sells, B. & Purdom, I. J. molec. Biol. 63, 21 (1972).
Granboulan, N. & Girard, M. J. Virol. 4, 475 (1969).
Schluederberg, A. E. Biochem. biophys. Res. Commun. 42, 1012 (1971).
Palacios, R., Palmiter, R. D. & Schimke, R. T. J. biol. Chem. 247, 2316 (1972).
Glisin, V., Crkvenjakov, R. & Byus, C. Biochemistry 13, 2633 (1974).
Viola, M. V. & White, L. Nature 246, 485 (1973).
Britten, R. & Kohne, D. Science 161, 529 (1968).
Vogt, V. Eur. J. Biochem. 33, 142 (1973).
Viola, M. V., Frazier, M., Wiernik, P., McCredie, K. & Spiegelman, S. New Engl. J. Med. 294, 75 (1976).
Ilyin, K., Bykovsky, A. & Zhdanov, V. Cancer 32, 89 (1973).
Rice, J. M. & Wolf, D. A. In Vitro 10, 352 (1975).
Hutton, J. & Wetmur, J. Biochem. biophys. Res. Commun. 52, 1148 (1973).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
VIOLA, M., GANN, K., SCOTT, C. et al. Absence of measles proviral DNA in systemic lupus erythematosus. Nature 275, 667–669 (1978). https://doi.org/10.1038/275667a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/275667a0
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.