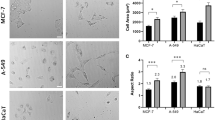
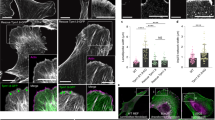
Abstract
ALTERATIONS in the surface membrane associated with transition of cells from the normal to the transformed state include changes in morphology1,2, membrane transport3, agglutination by plant lectins4,5, contact inhibition of growth6 and change in the lateral mobility of membrane components7. It has been suggested that these changes in surface properties are related to changes in the cytoskeleton. This idea is based on the concept that microfilaments and microtubules determine the topographical distribution and the mobility of membrane components and on the observation that in several cell types microfilament bundles disappear after transformation8–10. However, there is also evidence that the presence of microfilament bundles is related to adhesiveness to the substratum and is dissociable from cellular growth control11. We report here evidence for a difference in the cytoskeletal control of plasma membrane mobility between normal and transformed cells. We show that cytochalasin B (CB) induces polarisation of membrane components and actin and provokes a marked enhancement of capping in transformed but not in normal cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Porter, K. R., Todaro, G. J. & Fonte, V. J. Cell Biol. 59, 633–642 (1973).
Goldman, R. D., Chang, C. & Williams, J. F. Cold Spring Harb. Symp. quant. Biol. 39, 601–614 (1974).
Martin, G. S., Venuta, S., Weber, M. J. & Rubin, H. Proc. natn. Acad. Sci. U.S.A. 68, 2739–2741 (1971).
Talmadge, K. W., Noonan, K. D. & Burger, M. M. in Control of Proliferation in Animal Cells (eds Clarkson, B. & Baserga, R.) 313–327 (Cold Spring Harbor Laboratory, 1974).
Nicolson, G. L. Nature new Biol. 233, 244–246 (1971).
Abercrombie, M. & Heaysman, J. E. M. Expl Cell Res. 6, 293–306 (1954).
Nicolson, G. L. Biochim. biophys. Acta 458, 1–72 (1976).
Pollack, R., Osborn, M & Weber, K. Proc. natn. Acad. Sci. U.S.A. 72, 994–998 (1975).
Edelman, G. M. & Yahara, I. Proc. natn. Acad. Sci. U.S.A. 73, 2047–2051 (1976).
Ash, J. F., Vogt, P. K. & Singer, S. J. Proc. natn. Acad. Sci. U.S.A. 73, 3603–3607 (1976).
Willingham, M. C., Yamada, K. M., Yamada, S. S., Pouysségur, J. & Pastan, I. Cell 10, 375–380 (1977).
Willingham, M. C. & Pastan, I. J. Cell Biol. 67, 146–159 (1975).
Porter, K. R., Puck, T. T., Hsie, A. W. & Kelley, D. Cell 2, 145–152 (1974).
Puck, T. T. Proc. natn. Acad. Sci. U.S.A. 74, 449–452 (1977).
Sundqvist, K.-G. & Ehrnst, A. Nature 264, 226–231 (1976).
Wassarman, P. M., Ukena, T. E., Josefowicz, W. J., Letourneau, G. F. & Karnovsky, M. J. J. Cell Sci. 26, 323–337 (1977).
Brinkley, B. R., Fuller, G. M. & Highfield, D. P. Proc. natn. Acad. Sci. U.S.A. 72, 4981–4985 (1975).
Wickus, G., Gruenstein, E., Robbins, P. W. & Rich, A. Proc. natn. Acad. Sci. U.S.A. 72, 746–749 (1975).
Lidman, K. et al. Clin. exp. Immun. 24, 266–272 (1976).
Norberg, R. et al. Clin. exp. Immun. 28, 512–516 (1977).
Bergquist, N. R. & Schilling, W. G. E. E. Standardization in Immunofluorescence, 171–176 (Blackwell, Oxford, 1970).
Sundqvist, K.-G. Scand. J. Immun. 2, 479–494 (1973).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
SUNDQVIST, KG., OTTESKOG, P. & EGE, T. Cytochalasin B induces polarisation of plasma membrane components and actin in transformed cells. Nature 274, 915–917 (1978). https://doi.org/10.1038/274915a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/274915a0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.