Abstract
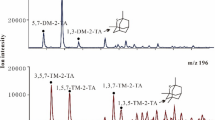
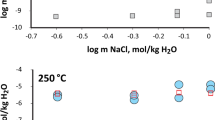
GREEN RIVER oil shale has been found to be converted to soluble products by the action of a sodium tetrachloroaluminate melt1. The function of the tetrachloroaluminate melt has been shown experimentally to include chemical effects in addition to its role as a thermal transfer agent. The results described here when compared to a conventional retorting process or a shift reaction2, are distinctly different, implying that the reaction could be thermally dependent, but results indicate the process involved is not a pyrolysis. Model studies have demonstrated that aromatic moieties are relatively inert to the solvent system described; however, aliphatic materials containing carbon in other than sp3 hybridisation are especially reactive. The mechanism of degradation seems to involve a mode of intramolecular disproportion catalysed by the tetrachloroaluminate melt's ability to stabilise the resulting shortlived intermediates until the macromolecules involved have been sufficiently reduced in size to become soluble in conventional solvents.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Koch, V. R., Miller, L. L. & Osteryoung, R. A. J. Am. chem. Soc. 98, 5277 (1976).
Boxall, L. G., Jones, H. L. & Osteryoung, R. A. J. electrochem. Soc. 120, 223 (1973).
Hubbard, A. B. & Robinson, W. E. Thermal Decomposition Study of Colorado Oil Shale (By Mines RI 4744, 1950).
Cummins, J. J. & Robinson, W. E. Thermal Degradation of Green River Kerogen at 150° to 350°C (By Mines RI 7620, 1972).
Robinson, W. E. & Cummins, J. J. An Oil Shale Conversion Process using Carbon Monoxide and Water (E.R.D.A. Techn. Progr. Rep. 75/1, 1975).
Lawlor, D. L., Fester, J. I. & Robinson, W. E. Fuel 40, 239–244 (1963).
Robinson, J., Gilbert, B. & Osteryoung, R. A. Inorg. Chem. 16, 3040 (1977).
Lawlor, D. L., Fester, J. I. & Robinson, W. E. Fuel 40, 239–244 (1963).
Bombi, G. G., Floriani, M. & Macca, C. Chem. Commun. 455 (1966).
Koch, V. R., Miller, L. L. & Osteryoung, R. A. J. Am. chem. Soc. 98, 5277 (1976).
Robinson, J. & Osteryoung, R. A. J. Am. chem. Soc. (submitted).
Anders, D. E., Cummins, J. J. & Robinson, W. E. Chem. Commun. 752 (1966).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
BUGLE, R., WILSON, K., OLSEN, G. et al. Oil-shale kerogen: low temperature degradation in molten salts. Nature 274, 578–580 (1978). https://doi.org/10.1038/274578a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/274578a0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.