Abstract
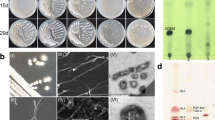
GAS vacuoles are subcellular inclusions in certain prokaryotic organisms which enclose a gas-filled space and consist of a proteinaceous membrane containing one or two very hydrophobic protein subunits1. Gas vacuoles are, with few exceptions, limited to aquatic microorganisms2, where it has been proposed either that they may be involved in regulating buoyancy, or that the light-scattering properties of the inclusions may protect the cells from the deleterious effects of high light intensities1. Several species of Halobacterium possess gas vacuoles3, and in certain strains, forms which lack gas vacuoles arise at a relatively high frequency. The rate of loss of gas vacuoles in Halobacterium strain 5 occurs so often that regular recloning of individual colonies containing gas vacuoles is necessary to maintain the wild-type strain4. Gas vacuoles are also lost spontaneously in other aquatic microorganisms, and it was this observation which led Walsby to propose that the gas-vacuole protein might be carried on a plasmid5. Plasmids have not previously been shown to exist in Halobacterium. All the obligate halophiles as yet examined contain a satellite DNA which may account for between 11 % and 36% of the total DNA (ref. 6, 7). Moore and McCarthy7, however, argued that the satellite DNA is not composed of multiple copies of a plasmid because its amount is unchanged by growing the cells in the presence of known plasmid-curing agents, and because total Halobacterium DNA renatures at a rate expected for single-copy DNA. Here I show that the wild-type Halobacterium strain 5 contains three plasmids, and also show a correlation between the presence of a plasmid of molecular weight (MW) 44×106 and the presence of gas vacuoles.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Walsby, A. E. Bact. Rev. 36, 1–32 (1972).
Cohen-Bazire, G., Kunisawa, R. & Pfennig, N. J. Bact. 100, 1049–1061 (1961).
Dundas, I. E. D. Adv. Microb. Physiol. 15, 85–120 (1977).
Larsen, H., Omang, S. & Steensland, H. Arch. Mikrobiol. 59, 197–203 (1967).
Walsby, A. E. Arch. Mikrobiol. 114, 167–170 (1977).
Joshi, J. G., Guild, W. R. & Handler, P. J. molec. Biol. 6, 34–38 (1963).
Moore, R. L. & McCarthy, B. J. J. Bact. 99, 248–254, 255–262 (1969).
Krantz, M. J. & Ballou, C. E. J. Bact. 114, 1058–1067 (1973).
Clewell, D. B. & Helinski, D. R. Proc. natn. Acad. Sci. U.S.A. 62, 1159–1166 (1969).
Meyers, J. A., Sanchez, D., Elwell, L. P. & Falkow, S. J. Bact. 127, 1529–1537 (1976).
Stanfield, S. & Helinski, D. R. Cell 9, 333–345 (1976).
Wheeler, F. C., Fishel, R. A. & Warner, R. C. Analyt. Biochem. 78, 260–275 (1977).
Keller, W. Proc. natn. Acad. Sci. U.S.A. 72, 4876–4880 (1975).
Espejo, R. T. & Lebowitz, J. Analyt. Biochem. 72, 95–103 (1976).
Wetmur, J. G. A. Rev. Biophys. Bioengng 5, 337–361 (1976).
Walsby, A. E. Arch. Microbiol. 109, 135–142 (1976).
Das, B. & Singh, P. K. Arch. Mikrobiol. 111, 195–196 (1976).
Walsby, A. E. Soc. gen. Microbiol. 28, 327–357 (1978).
Bedbrook, J. R. & Ausubel, F. M. Cell 9, 707–716 (1976).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
SIMON, R. Halobacterium strain 5 contains a plasmid which is correlated with the presence of gas vacuoles. Nature 273, 314–317 (1978). https://doi.org/10.1038/273314a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/273314a0
This article is cited by
-
Identification and analysis of the gas vesicle gene cluster on an unstable plasmid ofHalobacterium halobium
Experientia (1993)
-
Plasmid pHH1 of Halobacterium salinarium: characterization of the replicon region, the gas vesicle gene cluster and insertion elements
Molecular and General Genetics MGG (1993)
-
Tolerance of extremely halophilic archaebacteria towards bromide
Current Microbiology (1989)
-
Homologies between heterogeneous extrachromosomal DNA populations of Halobacterium halobium and four new halobacterial isolates
Molecular and General Genetics MGG (1984)
-
Lethality and mutagenicity inHalobacterium mediterranei caused by N-methyl-N′-nitro-N-nitrosoguanidine
Current Microbiology (1984)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.