Abstract
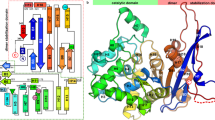
Bovine liver rhodanese is a single polypeptide of 293 amino acids in which the halves of the molecule assume analogous tertiary structures in the absence of substantial sequence homology. The sulphur atom transferred during catalysis is bound in persulphide linkage to Cys-247. Substrate binding seems to involve Arg-186 and Lys-249.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Roy, A. B. & Trudinger, P. A. The Biochemistry of Inorganic Compounds of Sulphur 190–206 (Cambridge University Press, 1970).
Westley, J. Adv. Enzymol. 39, 327–368 (1973).
Sörbo, B. H. in Metabolic Pathways Vol. 7 (ed. Greenberg, D. M.) 433–456 (Academic, New York, 1975).
Westley, J. in Bioorganic Chemistry (ed. Van Tamelen, E. E.) 371–390 (Academic, New York, 1977).
Westley, J. & Heyse, D. J. biol. Chem. 246, 1468–1474 (1971).
Sörbo, B. H. Acta chem. scand. 5, 724–734 (1951).
Sörbo, B. H. Acta chem. scand. 17, S107–S109 (1963).
Wang, S. F. & Volini, M. J. biol. Chem. 243, 5465–5470 (1968).
Mintel, R. & Westley, J. J. biol. Chem. 241, 3386–3389 (1966).
Davidson, B. & Westley, J. J. biol. Chem. 240, 4463–4469 (1965).
Horowitz, P. & Westley, J. J. biol. Chem. 245, 986–990 (1970).
Leininger, K. R. & Westley, J. J. biol. Chem. 243, 1892–1899 (1968).
Volini, M., DeToma, F. & Westley, J. J. biol. Chem. 242, 5220–5225 (1967).
Sörbo, B. H. Acta chem. scand. 7, 1129–1136 (1953).
Blumenthal, K. M. & Heinrikson, R. L. J. biol. Chem. 246, 2430–2437 (1971).
Green, J. R. & Westley, J. J. biol. Chem. 236, 3047–3050 (1961).
Westley, J. & Nakamoto, T. J. biol. Chem. 237, 547–549 (1962).
Volini, M. & Westley, J. J. biol. Chem. 241, 5168–5176 (1966).
Trumpower, B. L., Katki, A. & Horowitz, P. Biochem. biophys. Res. Commun. 57, 532–538 (1974).
Ellis, L. M. & Woodward, C. K. Biochim. biophys. Acta. 379, 385–396 (1975).
Crawford, J. M. & Horowitz, P. M. Biochim. biophys. Acta 429, 173–181 (1976).
Russell, J., Weng, L., Keim, P. S. & Heinrikson, R. L. Biochem. biophys. Res Commun. 64, 1090–1097 (1975).
Bergsma, J. et al. J. molec. Biol. 98, 637–643 (1975).
Horowitz, P. & DeToma, F. J. biol. Chem. 245, 984–985 (1970).
Heinrikson, R. L. & Kramer, K. J. Prog. Bioorg. Chem. 3, 141–250 (1974).
Ploegman, J. H. thesis, Univ. Groningen (1977).
Dickerson, R. E., Kendrew, J. C. & Strandberg, B. E. Acta Crystallogr. 14, 1188–1195 (1961).
Blow, D. M. & Crick, F. H. C. Acta Crystallogr., 12, 794–802 (1959).
Richards, F. M. J. molec. Biol. 37, 225–230 (1968).
Henderson, R. & Moffat, J. K. Acta Crystallogr. B 27, 1414–1420 (1971).
Bossa, F., Cannella, C., Federici, G., Barra, D. & Pecci, L. FEBS Lett. 29, 170–172 (1973).
Sternberg, M. J. E. & Thornton, J. M. J. molec. Biol. 110, 269–283 (1977).
Sternberg, M. J. E. & Thornton, J. M. J. molec. Biol. 105, 367–382 (1976).
Richardson, J. S. Proc. natn. Acad. Sci. U.S.A. 73, 2619–2623 (1976).
Rao, S. T. & Rossmann, M. G. J. molec. Biol. 76, 241–256 (1973).
Kretsinger, R. H. & Nockolds, C. E. J. biol. Chem. 248, 3313–3326 (1973).
Adman, E. T., Sieker, L. C. & Jensen, L. H. J. biol. Chem. 248, 3987–3996 (1973).
Quiocho, F. A., Gilliland, G. L. & Phillips, G. N. J. biol. Chem. 252, 5142–5149 (1977).
Rossmann, M. G., Liljas, A., Bränden, C. I. & Banaszak, L. J. in The Enzymes Vol. XI; 3rd edn (ed. Boyer, P. D.) 61–102 (Academic, New York, 1975).
Chou, P. Y. & Fasman, G. D. Biochemistry 13, 222–245 (1974).
Chou, P. Y. & Fasman, G. D. Adv. Enzymol. (in the press).
Burnett, R. M. et al. J. biol. Chem. 249, 4383–4392 (1974).
Eklund, H. et al. J. molec. Biol. 102, 27–59 (1976).
Weng, L. thesis, Univ. Chicago (1977).
Adman, E., Watenpaugh, K. D. & Jensen, L. H. Proc. natn. Acad. Sci. U.S.A. 72, 4854–4858 (1975).
Carter, C. W., Kraut, J., Freer, S. T. & Alden, R. A. J. biol. Chem. 249, 6339–6346 (1974).
Cannella, C., Pecci, L., Costa, M., Pensa, B. & Cavallini, D. Eur. J. Biochem. 56, 283–287 (1975).
Wang, S. F. & Volini, M. J. biol. Chem. 248, 7376–7385 (1973).
Volini, M. & Wang, S. F. J. biol. Chem. 248, 7386–7391 (1973).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ploegman, J., Drent, G., Kalk, K. et al. The covalent and tertiary structure of bovine liver rhodanese. Nature 273, 124–129 (1978). https://doi.org/10.1038/273124a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/273124a0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.