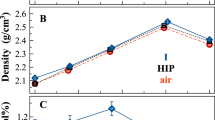
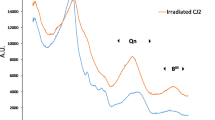
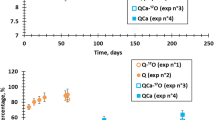
Abstract
WHEN a silicate glass comes into contact with a neutral or a slightly alkaline aqueous solution the only reaction which occurs is a diffusion controlled hydrogen–alkali ion exchange. The silica network is stable. In solutions with pH > about 9, the network starts to break down at its interface with the solution. The rate of breakdown increases sharply with increasing pH; simultaneously the ion exchange rate decreases sharply. In solutions with pH > about 12 it is difficult to detect the alkali released in solution by classical microanalytical methods as the solution is primarily made up of alkali hydroxide. It has been repeatedly suggested that the ion exchange reaction ceases to occur under these conditions and that the decomposition process becomes only a network breakdown. Surface and in-depth concentrations of alkalis in a glass which has been subjected to a highly alkaline solution have been determined by Auger electron spectroscopy (AES). Our results indicate that the ion exchange does not cease to occur in highly alkaline solutions as commonly believed.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
El-Shamy, T. M., Lewins, J. & Douglas, R. W. Glass Technol. 13, 81 (1972).
Pantano, C. G., Jr., Dove, D. B. & Onoda, G. Y., Jr. J. Noncryst. Solids 19, 41 (1975).
Tsuchihashi, S. & Sekido, E. Bull chem. Soc. Japan 32, 868 (1959).
Hench, L. L. Noncryst. J. Solids 19, 27 (1975).
Isard, J. O. Glass Electrodes for Hydrogen and Other Cations: Principles and Practice (ed. Eisenman, G.) (Dekker, New York, 1967).
Dubrovo, S. K. and Shmidt, Y. A. Bull. Acad. Sci. USSR Div. chem. Sci. 403 (C. B. translation, 1958).
Chang, C. C. Analytical Auger Spectroscopy: Characterization of Solid Surfaces (Plenum, New York, 1974).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
EL-SHAMY, T., PANTANO, C. Decomposition of silicate glasses in alkaline solutions. Nature 266, 704–706 (1977). https://doi.org/10.1038/266704a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/266704a0
This article is cited by
-
A Review on Waste Glass-based Geopolymer Composites as a Sustainable Binder
Silicon (2023)
-
Influence of coated surfaces and gap size on boiling heat transfer of deionized water
Journal of the Brazilian Society of Mechanical Sciences and Engineering (2020)
-
Enhanced stability of a Prussian blue/sol-gel composite for electrochemical determination of hydrogen peroxide
Microchimica Acta (2014)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.