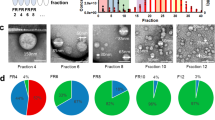
Abstract
WE have already presented preliminary evidence1 that crude plasma from tumour-bearing rats contains elevated concentrations of macromolecules which stimulate the release of messenger RNA (mRNA) from isolated nuclei. The detection of these putative regulatory components was facilitated by a cell-free system developed originally to study messenger2–5 and ribosomal6,7 RNA (rRNA) processing and transport in vitro. Since both maximal RNA transport and normal nuclear RNA restriction in vitro (that is, equivalence between messengers released from nuclei in vivo and in vitro) require the presence of macromolecules from homologous cytosol2,8 it is possible that the tumour cell cytoplasm is the source of the putative regulatory macromolecules in the peripheral blood of tumour-bearing animals. This possibility is supported by evidence9,10 that factors involved in the regulation of cellular proliferation are released to circulation from tumour cells, and by the observation11 that the enzyme composition of the host liver of tumour-bearing rats converges to that of the tumour. The spectrum of messengers transported from normal rat liver nuclei in homologous cytosol is significantly different from that transported in hepatoma cytosol3, suggesting that differences exist in these putative regulatory components of the two tissues.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Schumm, D. E., and Webb, T. E., J. natn. Cancer Inst., 54, 123–128 (1975).
Schumm, D. E., and Webb, T. E., Biochem. biophys. Res. Commun., 48, 1259–1265 (1972).
Schumm, D. E., Morris, H. P., and Webb, T. E., Cancer Res., 33, 1821–1828 (1973).
Schumm, D. E., McNamara, D. J., and Webb, T. E., Nature new Biol., 245, 201–203 (1973).
Schumm, D. E., and Webb, T. E., Biochem. J., 139, 191–196 (1974).
Yu, L. C., Racevskis, J., and Webb, T. E., Cancer Res., 32, 2314–2321 (1971).
Racevskis, J., and Webb, T. E., Eur. J. Biochem., 49, 93–100 (1974).
Schumm, D. E., and Webb, T. E., Biochem. biophys. Res. Commun., 58, 354–360 (1974).
Bullough, W. S., and Lawrence, E. B., Nature, 220, 137–138 (1968).
Rytoma, T., and Kiviniemk, K., Nature, 222, 995–996 (1969).
Herzfeld, A., and Greengard, O., Cancer Res., 32, 1826–1832 (1972).
Morris, H. P., and Wagner, B. P., in Methods in Cancer Research, 4 (edit. by Busch, H.), 125–137 (Academic, New York, 1968).
Drews, J., Brawerman, G., and Morris, H. P., Eur. J. Biochem., 3, 284–292 (1968).
Birnie, B. D., Proc. XIth Int. Cancer Congress, Florence (in the press).
Sporn, M. B., Biochem. Pharm., 20, 1029 (1971).
Dolan, M. L., Coetzee, M. L., Spangler, M., and Ove, P., Cancer Res., 34, 3010–3017 (1974).
Ove, P., Coetzee, M. L., Obenrader, M., and Short, T., Oncology, 29, 13–21 (1974).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
SCHUMM, D., WEBB, T. Differential effect of plasma fractions from normal and tumour-bearing rats on nuclear RNA restriction. Nature 256, 508–509 (1975). https://doi.org/10.1038/256508a0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/256508a0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.