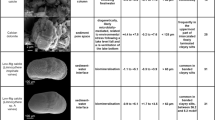
Abstract
SUPERFICIAL carbonate deposits are common on bedrock surfaces recently exposed by retreating temperate glaciers1–5. Their morphology indicates that they formed subglacially in intimate contact with sliding ice. Besides suggesting that chemical transport is an active subglacial process, these relatively widespread deposits reflect high solute concentrations in subglacial waters. This is a fact of considerable significance because solutes at the glacier–bed interface can impede significantly the sliding of temperate glaciers5. The dynamics of temperate glaciers and especially the more intriguing aspects of their behaviour, such as surging6, are thought to depend critically on the basal sliding process7. Thus, chemical exchange at the base of a glacier may affect significantly its entire motion. Moreover, because glacial erosion and deposition are largely dependent on glacial sliding, they too are affected by the presence of solutes at the bed. To date, reported subglacial deposits have all been carbonates. On the basis of previously unrecognised subglacial deposits rich in silica, I suggest that subglacial deposits are not exclusively carbonates, but that silicates are of wider significance, than is believed at present.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Ford, D. C., Fuller, P. G., and Drake, J. J., Nature, 226, 441–442 (1970).
Page, N. R., Nature, 229, 42–43 (1971).
Kers, L. E., Geol. För. Stockh. Förh., 86, 282–309 (1964).
Bauer, V. G., Z. Gletscherk. Glazialgeol., 4 (3), 215–225 (1961).
Hallet, B., Bull. geol. Soc. Am. (in the press).
Budd, W. F., and McInnes, B. J., Science, 186, 4167, 925–927 (1974).
Paterson, W. S. B., J. Glaciol., 9 (55), 55–63 (1970).
Nye, J. F., Proc. R. Soc., A 311, 445–467 (1969).
Kamb, B., Rev. Geophys. Space Phys., 8 (4), 673–728 (1970).
Terwilliger, J. P., and Dizio, S. F., Chem. Engng Sci., 25, 1331–1349 (1970).
Kvajic, G., and Brajovic, V., J. Cryst. Growth, 11 (1), 73–76 (1971).
Seidensticker, R. G., J. chem. Phys., 56 (6), 2853–2857 (1972).
Keller, W. D., and Reesman, A. L., Bull. geol. Soc. Am., 74, 61–76 (1963).
Sigafoos, R. S., and Hendricks, E. L., Prof. Pap. US geol. Surv., 387, B1–B24 (1972).
Krauskopf, K., Geochim. cosmochim. Acta, 10, 1–26 (1956).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
HALLET, B. Subglacial silica deposits. Nature 254, 682–683 (1975). https://doi.org/10.1038/254682a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/254682a0
This article is cited by
-
Ice retreat in Wilkes Basin of East Antarctica during a warm interglacial
Nature (2020)
-
Mineral deformation and subglacial processes on ice-bedrock interface of Hailuogou Glacier
Chinese Science Bulletin (2009)
-
Characteristics of the subglacially-formed debris-rich chemical deposits and related subglacial processes of Qiangyong Glacier, Tibet
Journal of Geographical Sciences (2003)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.