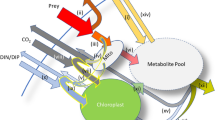
Abstract
Iron is unique among biologically essential trace metals in having a higher particulate than dissolved concentration in ocean surface waters1. Uptake of dissolved iron is generally considered to be the norm for phytoplankton, as even the smallest iron-bearing particles are unavailable for transport into cells2,3. But the oceanic dissolved fraction is so small, and the particulate fraction so inert2, that phytoplankton production is limited by a dearth of available iron in some regions4. Here we use incubation experiments to show that Ochromonas sp., a common photosynthetic flagellate from the Pacific Ocean, can obtain iron directly in particulate form, by ingesting bacteria. Iron acquisition is highly efficient; Ochromonas assimilates 30% of the ingested ration, acquiring a high intracellular iron concentration and maintaining a significantly faster growth rate than when iron is provided in the dissolved phase. Phytoplankton capable of such phagotrophy (so-called mixotrophic species) may thus be able to assimilate iron in both particulate and dissolved forms in the ocean. Moreover, when iron availability is limited, the iron ‘cost’ of growth is diminished because Ochromonas derives a greater fraction of its energy from the bacteria. Analysis of standing stocks and clearance rates of plankton in the equatorial Pacific shows that the iron flux through mixotrophic flagellates can amount to 35–58% of the total Fe uptake by the entire autotrophic community. Our results suggest that the phagotrophic ingestion of bacteria may be an effective adaptive strategy for photosynthetic organisms to obtain iron for growth in iron-limited regions of the sea.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Johnson, K. S., Gordon, R. M. & Coale, K. H. What controls dissolved iron concentrations in the world ocean? Mar. Chem. 57, 137–161 (1997).
Rich, H. W. & Morel, F. M. M. Availability of well-defined iron colloids to the marine diatom Thalassiosira weissflogii. Limnol. Oceanogr. 35, 652–662 (1990).
Price, N. M. & Morel, F. M. M. in Iron Transport and Storage in Microorganisms Plants and Animals: Metal Ions in Biological Systems (eds Sigel, A. & Sigel, H.) 1–36 (Decker, New York, 1998).
Coale, K. H. et al. Amassive phytoplankton bloom induced by an ecosystem-scale iron fertilisation experiment in the equatorial Pacific. Nature 383, 495–501 (1996).
Hudson, R. J. M. & Morel, F. M. M. Iron transport in marine phytoplankton: Kinetics of cellular and medium control reactions. Limnol. Oceanogr. 35, 1002–1020 (1990).
Maldonado, M. T. & Price, N. M. Utilization of iron bound to strong organic ligands by plankton communities in the subarctic Pacific Ocean. Deep-Sea Res. (in the press).
Rothhaupt, K. O. Utilization of substitutable carbon and phosphorus sources by the mixotrophic chrysophyte Ochromonas sp. Ecology 77, 706–715 (1996).
Caron, D. A. et al. Light-dependent phagotrophy in the freshwater mixotrophic chrysophyte Dinobyron cylindricum. Microb. Ecol. 25, 93–111 (1993).
Raven, J. A. Phagotrophy in phototrophs. Limnol. Oceanogr. 42, 198–205 (1997).
Tortell, P. D., Maldonado, M. T. & Price, N. M. The role of heterotrophic bacteria in iron-limited ocean ecosystems. Nature 383, 330–332 (1996).
Chase, Z. & Price, N. M. Metabolic consequences of Fe deficiency in heterotrophic marine protozoa. Limnol. Oceanogr. 42, 1673–1684 (1997).
Hutchins, D. A., DiTullio, G. R. & Bruland, K. W. Iron and regenerated production—evidence for biological iron recycling in two marine environments. Limnol. Oceanogr. 38, 1242–1255 (1993).
Sunda, W. G. & Huntsman, S. A. Iron uptake and growth limitation in oceanic and coastal phytoplankton. Mar. Chem. 50, 189–206 (1995).
Raven, J. A. The iron and molybdenum use efficiencies of plant growth with different energy, carbon and nitrogen sources. New Phytol. 116, 1–18 (1988).
Maldonado, M. T. & Price, N. M. Influence of N substrate on Fe requirements of marine centric diatoms. Mar. Ecol. Prog. Ser. 141, 161–172 (1996).
Barbeau, K., Moffett, J. W., Caron, D. A., Croot, P. L. & Erdner, D. L. Role of protozoan grazing in relieving iron limitation of phytoplankton. Nature 380, 61–64 (1996).
Twiss, M. R. & Campbell, P. G. C. Regeneration of trace metals from picoplankton by nanoflagellate grazing. Limnol. Oceanogr. 40, 1418–1429 (1995).
Sanders, R. W. Mixotrophic protists in marine and freshwater ecosystems. J. Protozool. 38, 76–81 (1991).
La Roche, J., Boyd, P. W., McKay, M. L. & Geider, R. J. Flavodoxin as an in situ marker for iron stress in phytoplankton. Nature 382, 802–805 (1996).
Chavez, F. P., Buck, K. R., Service, S. K., Newton, J. & Barber, R. T. Phytoplankton variability in the central and eastern tropical Pacific. Deep Sea Res. II 43, 835–870 (1996).
Strøm, S. & Welschmeyer, N. A. Pigment-specific rates of phytoplankton growth and microzooplankton grazing in the open subarctic Pacific Ocean. Limnol. Oceanogr. 36, 50–63 (1991).
Constantinou, J. Mixotrophic Nanoflagellates in Marine Microbial Food Webs. Thesis, Univ. Hawaii (1994).
Stoecker, D. K., Gustason, D. E. & Verity, P. G. Micro- and mesoprotozooplankton at 140° W in the equatorial Pacific: heterotrophs and mixotrophs. Aquat. Microb. Ecol. 10, 273–282 (1996).
Price, N. M. et al. Preparation and chemistry of the artificial algal culture media Aquil. Biol. Oceanogr. 6, 443–462 (1988/89).
Hudson, R. J. M. & Morel, F. M. M. Distinguishing between extra- and intracellular iron in marine phytoplankton. Limnol. Oceanogr. 34, 1113–1120 (1989).
Børsheim, K. Y. & Bratbak, G. Cell volume to cell carbon conversion factors for a bacterivorous Monas sp. enriched from seawater. Mar. Ecol. Prog. Ser. 36, 171–175 (1987).
Peters, F. The prediction of planktonic protistan grazing rates. Limnol. Oceanogr. 39, 195–205 (1994).
Bjørnsen, P. K. & Kuparinen, J. Growth and herbivory by heterotrophic dinoflagellates in the Southern Ocean, studied by mesocosm experiments. Mar. Biol. 109, 397–405 (1991).
Barber, R. T. et al. Primary productivity and its regulation in the equatorial Pacific during and following the 1991–1992 El Niño. Deep Sea Res. II 43, 933–969 (1996).
Binder, B. J., Chisholm, S. W., Olson, R. J., Frankel, S. L. & Worden, A. Z. Dynamics of picophytoplankton, ultraphytoplankton and bacteria in the central equatorial Pacific. Deep-Sea Res. II 43, 907–931 (1996).
McCarthy, J. J., Garside, C., Nevins, J. L. & Barber, R. T. New production along the 140° W in the equatorial Pacific during and following an El Niño event. Deep-Sea Res. II 43, 1065–1093 (1996).
Acknowledgements
We thank M. T. Maldonado and J. Granger for technical advice and comments on the manuscript, and D. M. Karl for discussion. Technical assistance was provided by P. Fenwick and A.Soucisse. R.M. was supported by a FCAR (Quebec) student scholarship. This work was funded by NSERC (Canada) grants to D.F.B. and N.M.P., and by the McGill Faculty of Graduate Studies and Research. This is a contribution to the Canadian JGOFS program and to the GRIL.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Maranger, R., Bird, D. & Price, N. Iron acquisition by photosynthetic marine phytoplankton from ingested bacteria. Nature 396, 248–251 (1998). https://doi.org/10.1038/24352
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/24352
This article is cited by
-
Importance of mixotrophic flagellates during the ice-free season in lakes located along an elevational gradient
Aquatic Sciences (2019)
-
Pelagibaca bermudensis promotes biofuel competence of Tetraselmis striata in a broad range of abiotic stressors: dynamics of quorum-sensing precursors and strategic improvement in lipid productivity
Biotechnology for Biofuels (2018)
-
Distribution of mixotrophic nanoflagellates along the latitudinal transect of the central North Pacific
Journal of Oceanography (2017)
-
A light-induced shortcut in the planktonic microbial loop
Scientific Reports (2016)
-
Physiological Responses of Three Species of Antarctic Mixotrophic Phytoflagellates to Changes in Light and Dissolved Nutrients
Microbial Ecology (2015)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.