Abstract
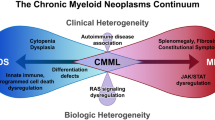
The 2001 World Health Organization (WHO) treatise on the classification of hematopoietic tumors lists chronic myeloproliferative diseases (CMPDs) as a subdivision of myeloid neoplasms that includes the four classic myeloproliferative disorders (MPDs)—chronic myelogenous leukemia, polycythemia vera (PV), essential thrombocythemia (ET) and primary myelofibrosis (PMF)—as well as chronic neutrophilic leukemia (CNL), chronic eosinophilic leukemia/hypereosinophilic syndrome (CEL/HES) and ‘CMPD, unclassifiable’. In the upcoming 4th edition of the WHO document, due out in 2008, the term ‘CMPDs’ is replaced by ‘myeloproliferative neoplasms (MPNs)’, and the MPN category now includes mast cell disease (MCD), in addition to the other subcategories mentioned above. At the same time, however, myeloid neoplasms with molecularly characterized clonal eosinophilia, previously classified under CEL/HES, are now removed from the MPN section and assembled into a new category of their own. The WHO diagnostic criteria for both the classic BCR–ABL-negative MPDs (that is PV, ET and PMF) and CEL/HES have also been revised, in the 2008 edition, by incorporating new information on their molecular pathogenesis. The current review highlights these changes and also provides diagnostic algorithms that are tailored to routine clinical practice.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Dameshek W . Some speculations on the myeloproliferative syndromes. Blood 1951; 6: 372–375.
Vardiman JW, Harris NL, Brunning RD . The World Health Organization (WHO) classification of the myeloid neoplasms. Blood 2002; 100: 2292–2302.
Jaffe ES, Harris NL, Stein H, Vardiman JW . World Health Organization Classification of Tumours of Hematopoietic and Lymphoid Tissues. IARC Press: Lyon, France, 2001, pp 1–351.
Vardiman JW, Brunning RD, Harris NL . WHO histological classification of chronic myeloproliferative diseases. In: Jaffe ES, Harris NL, Stein H, Vardiman JW (eds). World Health Organization Classification of Tumors: Tumours of the Haematopoietic and Lymphoid Tissues. International Agency for Research on Cancer (IARC) Press: Lyon, France, 2001, 17–44.
Fialkow PJ . Cell lineages in hematopoietic neoplasia studied with glucose-6-phosphate dehydrogenase cell markers. J Cell Physiol Suppl 1982; 1: 37–43.
Tefferi A, Gilliland DG . Oncogenes in myeloproliferative disorders. Cell Cycle 2007; 6: 550–566.
De Keersmaecker K, Cools J . Chronic myeloproliferative disorders: a tyrosine kinase tale. Leukemia 2006; 20: 200–205.
Baxter EJ, Scott LM, Campbell PJ, East C, Fourouclas N, Swanton S et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet 2005; 365: 1054–1061.
Levine RL, Wadleigh M, Cools J, Ebert BL, Wernig G, Huntly BJ et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell 2005; 7: 387–397.
Kralovics R, Passamonti F, Buser AS, Teo SS, Tiedt R, Passweg JR et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med 2005; 352: 1779–1790.
James C, Ugo V, Le Couedic JP, Staerk J, Delhommeau F, Lacout C et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature 2005; 434: 1144–1148.
Scott LM, Tong W, Levine RL, Scott MA, Beer PA, Stratton MR et al. JAK2 exon 12 mutations in polycythemia vera and idiopathic erythrocytosis. N Engl J Med 2007; 356: 459–468.
Pardanani A, Lasho TL, Finke C, Hanson CA, Tefferi A . Prevalence and clinicopathologic correlates of JAK2 exon 12 mutations in JAK2V617F-negative polycythemia vera. Leukemia 2007; 21: 1960–1963.
Pikman Y, Lee BH, Mercher T, McDowell E, Ebert BL, Gozo M et al. MPLW515L is a novel somatic activating mutation in myelofibrosis with myeloid metaplasia. PLoS Med 2006; 3: e270.
Pardanani AD, Levine RL, Lasho T, Pikman Y, Mesa RA, Wadleigh M et al. MPL515 mutations in myeloproliferative and other myeloid disorders: a study of 1182 patients. Blood 2006; 108: 3472–3476.
Cools J, DeAngelo DJ, Gotlib J, Stover EH, Legare RD, Cortes J et al. A tyrosine kinase created by fusion of the PDGFRA and FIP1L1 genes as a therapeutic target of imatinib in idiopathic hypereosinophilic syndrome. N Engl J Med 2003; 348: 1201–1214.
Pardanani A, Brockman SR, Paternoster SF, Flynn HC, Ketterling RP, Lasho TL et al. FIP1L1-PDGFRA fusion: prevalence and clinicopathologic correlates in 89 consecutive patients with moderate to severe eosinophilia. Blood 2004; 104: 3038–3045.
Golub TR, Barker GF, Lovett M, Gilliland DG . Fusion of PDGF receptor beta to a novel ets-like gene, tel, in chronic myelomonocytic leukemia with t(5;12) chromosomal translocation. Cell 1994; 77: 307–316.
Xiao S, Nalabolu SR, Aster JC, Ma J, Abruzzo L, Jaffe ES et al. FGFR1 is fused with a novel zinc-finger gene, ZNF198, in the t(8;13) leukaemia/lymphoma syndrome. Nat Genet 1998; 18: 84–87.
Bennett JM . A comparative review of classification systems in myelodysplastic syndromes (MDS). Semin Oncol 2005; 32: S3–S10.
Bennett JM, Catovsky D, Daniel MT, Flandrin G, Galton DA, Gralnick H et al. The chronic myeloid leukaemias: guidelines for distinguishing chronic granulocytic, atypical chronic myeloid, and chronic myelomonocytic leukaemia. Proposals by the French–American–British Cooperative Leukaemia Group. Br J Haematol 1994; 87: 746–754.
Schmitt-Graeff A, Thiele J, Zuk I, Kvasnicka HM . Essential thrombocythemia with ringed sideroblasts: a heterogeneous spectrum of diseases, but not a distinct entity. Haematologica 2002; 87: 392–399.
Cabello AI, Collado R, Ruiz MA, Martinez J, Navarro I, Ferrer R et al. A retrospective analysis of myelodysplastic syndromes with thrombocytosis: reclassification of the cases by WHO proposals. Leuk Res 2005; 29: 365–370.
Fialkow PJ, Gartler SM, Yoshida A . Clonal origin of chronic myelocytic leukemia in man. Proc Natl Acad Sci USA 1967; 58: 1468–1471.
Adamson JW, Fialkow PJ, Murphy S, Prchal JF, Steinmann L . Polycythemia vera: stem-cell and probable clonal origin of the disease. N Engl J Med 1976; 295: 913–916.
Jacobson RJ, Salo A, Fialkow PJ . Agnogenic myeloid metaplasia: a clonal proliferation of hematopoietic stem cells with secondary myelofibrosis. Blood 1978; 51: 189–194.
Fialkow PJ, Faguet GB, Jacobson RJ, Vaidya K, Murphy S . Evidence that essential thrombocythemia is a clonal disorder with origin in a multipotent stem cell. Blood 1981; 58: 916–919.
Robyn J, Lemery S, McCoy JP, Kubofcik J, Kim YJ, Pack S et al. Multilineage involvement of the fusion gene in patients with FIP1L1/PDGFRA-positive hypereosinophilic syndrome. Br J Haematol 2006; 132: 286–292.
Tefferi A, Lasho TL, Brockman SR, Elliott MA, Dispenzieri A, Pardanani A . FIP1L1-PDGFRA and c-kit D816 V mutation-based clonality studies in systemic mast cell disease associated with eosinophilia. Haematologica 2004; 89: 871–873.
Bohm J, Kock S, Schaefer HE, Fisch P . Evidence of clonality in chronic neutrophilic leukaemia. J Clin Pathol 2003; 56: 292–295.
Froberg MK, Brunning RD, Dorion P, Litz CE, Torlakovic E . Demonstration of clonality in neutrophils using FISH in a case of chronic neutrophilic leukemia. Leukemia 1998; 12: 623–626.
Yanagisawa K, Ohminami H, Sato M, Takada K, Hasegawa H, Yasukawa M et al. Neoplastic involvement of granulocytic lineage, not granulocytic–monocytic, monocytic, or erythrocytic lineage, in a patient with chronic neutrophilic leukemia. Am J Hematol 1998; 57: 221–224.
Chang HW, Leong KH, Koh DR, Lee SH . Clonality of isolated eosinophils in the hypereosinophilic syndrome. Blood 1999; 93: 1651–1657.
Akin C, Kirshenbaum AS, Semere T, Worobec AS, Scott LM, Metcalfe DD . Analysis of the surface expression of c-kit and occurrence of the c-kit Asp816Val activating mutation in T cells, B cells, and myelomonocytic cells in patients with mastocytosis. Exp Hematol 2000; 28: 140–147.
Yavuz AS, Lipsky PE, Yavuz S, Metcalfe DD, Akin C . Evidence for the involvement of a hematopoietic progenitor cell in systemic mastocytosis from single-cell analysis of mutations in the c-kit gene. Blood 2002; 100: 661–665.
Taylor ML, Sehgal D, Raffeld M, Obiakor H, Akin C, Mage RG et al. Demonstration that mast cells, T cells, and B cells bearing the activating kit mutation D816V occur in clusters within the marrow of patients with mastocytosis. J Mol Diagn 2004; 6: 335–342.
Wasserman LR . The treatment of polycythemia. A panel discussion. Blood 1968; 32: 483–487.
Murphy S, Iland H, Rosenthal D, Laszlo J . Essential thrombocythemia: an interim report from the Polycythemia Vera Study Group. Semin Hematol 1986; 23: 177–182.
Tefferi A, Thiele J, Orazi A, Kvasnicka HM, Barbui T, Hanson CA et al. Proposals and rationale for revision of the World Health Organization diagnostic criteria for polycythemia vera, essential thrombocythemia, and primary myelofibrosis: recommendations from an ad hoc international expert panel. Blood 2007; 110: 1092–1097.
Wong CL, Ma ES, Wang CL, Lam HY, Ma SY . JAK2 V617F due to a novel TG → CT mutation at nucleotides 1848–1849: diagnostic implication. Leukemia 2007; 21: 1344–1346.
Grunebach F, Bross-Bach U, Kanz L, Brossart P . Detection of a new JAK2 D620E mutation in addition to V617F in a patient with polycythemia vera. Leukemia 2006; 20: 2210–2211.
Verstovsek S, Silver RT, Cross NC, Tefferi A . JAK2V617F mutational frequency in polycythemia vera: 100%, >90%, less? Leukemia 2006; 20: 2067.
Tefferi A, Strand JJ, Lasho TL, Knudson RA, Finke CM, Gangat N et al. Bone marrow JAK2V617F allele burden and clinical correlates in polycythemia vera. Leukemia 2007; 21: 2074–2075.
Tefferi A . JAK2 mutations in myeloproliferative disorders—molecular mechanisms and clinical applications. N Engl J Med 2007; 356: 444–445.
Levine RL, Belisle C, Wadleigh M, Zahrieh D, Lee S, Chagnon P et al. X-inactivation-based clonality analysis and quantitative JAK2V617F assessment reveal a strong association between clonality and JAK2V617F in PV but not ET/MMM, and identifies a subset of JAK2V617F-negative ET and MMM patients with clonal hematopoiesis. Blood 2006; 107: 4139–4141.
Melzner I, Weniger MA, Menz CK, Moller P . Absence of the JAK2 V617F activating mutation in classical Hodgkin lymphoma and primary mediastinal B-cell lymphoma. Leukemia 2006; 20: 157–158.
McClure RF, Hoyer JD, Mai M . The JAK2 V617F mutation is absent in patients with erythrocytosis due to high oxygen affinity hemoglobin variants. Hemoglobin 2006; 30: 487–489.
Tefferi A, Sirhan S, Lasho TL, Schwager SM, Li CY, Dingli D et al. Concomitant neutrophil JAK2 mutation screening and PRV-1 expression analysis in myeloproliferative disorders and secondary polycythaemia. Br J Haematol 2005; 131: 166–171.
Antonioli E, Guglielmelli P, Pancrazzi A, Bogani C, Verrucci M, Ponziani V et al. Clinical implications of the JAK2 V617F mutation in essential thrombocythemia. Leukemia 2005; 19: 1847–1849.
Wolanskyj AP, Lasho TL, Schwager SM, McClure RF, Wadleigh M, Lee SJ et al. JAK2 mutation in essential thrombocythaemia: clinical associations and long-term prognostic relevance. Br J Haematol 2005; 131: 208–213.
Vizmanos JL, Ormazabal C, Larrayoz MJ, Cross NC, Calasanz MJ . JAK2 V617F mutation in classic chronic myeloproliferative diseases: a report on a series of 349 patients. Leukemia 2006; 20: 534–535.
Campbell PJ, Scott LM, Buck G, Wheatley K, East CL, Marsden JT et al. Definition of subtypes of essential thrombocythaemia and relation to polycythaemia vera based on JAK2 V617F mutation status: a prospective study. Lancet 2005; 366: 1945–1953.
Kittur J, Knudson RA, Lasho TL, Finke CM, Gangat N, Wolanskyj AP et al. Clinical correlates of JAK2V617F allele burden in essential thrombocythemia. Cancer 2007; 109: 2279–2284.
Heller PG, Lev PR, Salim JP, Kornblihtt LI, Goette NP, Chazarreta CD et al. JAK2V617F mutation in platelets from essential thrombocythemia patients: correlation with clinical features and analysis of STAT5 phosphorylation status. Eur J Haematol 2006; 77: 210–216.
Tefferi A, Lasho TL, Schwager SM, Steensma DP, Mesa RA, Li CY et al. The JAK2 tyrosine kinase mutation in myelofibrosis with myeloid metaplasia: lineage specificity and clinical correlates. Br J Haematol 2005; 131: 320–328.
Campbell PJ, Griesshammer M, Dohner K, Dohner H, Kusec R, Hasselbalch HC et al. V617F mutation in JAK2 is associated with poorer survival in idiopathic myelofibrosis. Blood 2006; 107: 2098–2100.
Szpurka H, Tiu R, Murugesan G, Aboudola S, Hsi ED, Theil KS et al. Refractory anemia with ringed sideroblasts associated with marked thrombocytosis (RARS-T), another myeloproliferative condition characterized by JAK2 V617F mutation. Blood 2006; 108: 2173–2181.
Ceesay MM, Lea NC, Ingram W, Westwood NB, Gaken J, Mohamedali A et al. The JAK2 V617F mutation is rare in RARS but common in RARS-T. Leukemia 2006; 20: 2060–2061.
Wang SA, Hasserjian RP, Loew JM, Sechman EV, Jones D, Hao S et al. Refractory anemia with ringed sideroblasts associated with marked thrombocytosis harbors JAK2 mutation and shows overlapping myeloproliferative and myelodysplastic features. Leukemia 2006; 20: 1641–1644.
Renneville A, Quesnel B, Charpentier A, Terriou L, Crinquette A, Lai JL et al. High occurrence of JAK2 V617 mutation in refractory anemia with ringed sideroblasts associated with marked thrombocytosis. Leukemia 2006; 20: 2067–2070.
Remacha AF, Nomdedeu JF, Puget G, Estivill C, Sarda MP, Canals C et al. Occurrence of the JAK2 V617F mutation in the WHO provisional entity: myelodysplastic/myeloproliferative disease, unclassifiable-refractory anemia with ringed sideroblasts associated with marked thrombocytosis. Haematologica 2006; 91: 719–720.
Zecca M, Bergamaschi G, Kratz C, Bergstrasser E, Danesino C, De Filippi P et al. JAK2 V617F mutation is a rare event in juvenile myelomonocytic leukemia. Leukemia 2007; 21: 367–369.
Kremer M, Horn T, Dechow T, Tzankov A, Quintanilla-Martinez L, Fend F . The JAK2 V617F mutation occurs frequently in myelodysplastic/myeloproliferative diseases, but is absent in true myelodysplastic syndromes with fibrosis. Leukemia 2006; 20: 1315–1316.
Nishii K, Nanbu R, Lorenzo VF, Monma F, Kato K, Ryuu H et al. Expression of the JAK2 V617F mutation is not found in de novo AML and MDS but is detected in MDS-derived leukemia of megakaryoblastic nature. Leukemia 2007; 21: 1337–1338.
Jelinek J, Oki Y, Gharibyan V, Bueso-Ramos C, Prchal JT, Verstovsek S et al. JAK2 mutation 1849G>T is rare in acute leukemias but can be found in CMML, Philadelphia chromosome-negative CML, and megakaryocytic leukemia. Blood 2005; 106: 3370–3373.
Steensma DP, McClure RF, Karp JE, Tefferi A, Lasho TL, Powell HL et al. JAK2 V617F is a rare finding in de novo acute myeloid leukemia, but STAT3 activation is common and remains unexplained. Leukemia 2006; 20: 971–978.
Lee JW, Kim YG, Soung YH, Han KJ, Kim SY, Rhim HS et al. The JAK2 V617F mutation in de novo acute myelogenous leukemias. Oncogene 2006; 25: 1434–1436.
Frohling S, Lipka DB, Kayser S, Scholl C, Schlenk RF, Dohner H et al. Rare occurrence of the JAK2 V617F mutation in AML subtypes M5, M6, and M7. Blood 2006; 107: 1242–1243.
Steensma DP, Dewald GW, Lasho TL, Powell HL, McClure RF, Levine RL et al. The JAK2 V617F activating tyrosine kinase mutation is an infrequent event in both ‘atypical’ myeloproliferative disorders and myelodysplastic syndromes. Blood 2005; 106: 1207–1209.
Jones AV, Kreil S, Zoi K, Waghorn K, Curtis C, Zhang L et al. Widespread occurrence of the JAK2 V617F mutation in chronic myeloproliferative disorders. Blood 2005; 106: 2162–2168.
Fiorini A, Farina G, Reddiconto G, Palladino M, Rossi E, Za T et al. Screening of JAK2 V617F mutation in multiple myeloma. Leukemia 2006; 20: 1912–1913.
Levine RL, Loriaux M, Huntly BJ, Loh ML, Beran M, Stoffregen E et al. The JAK2V617F activating mutation occurs in chronic myelomonocytic leukemia and acute myeloid leukemia, but not in acute lymphoblastic leukemia or chronic lymphocytic leukemia. Blood 2005; 106: 3377–3379.
Sulong S, Case M, Minto L, Wilkins B, Hall A, Irving J . The V617F mutation in Jak2 is not found in childhood acute lymphoblastic leukaemia. Br J Haematol 2005; 130: 964–965.
Sidon P, El Housni H, Dessars B, Heimann P . The JAK2V617F mutation is detectable at very low level in peripheral blood of healthy donors. Leukemia 2006; 20: 1622.
Lengfelder E, Hochhaus A, Kronawitter U, Hoche D, Queisser W, Jahn-Eder M et al. Should a platelet limit of 600 × 10(9)/l be used as a diagnostic criterion in essential thrombocythaemia? An analysis of the natural course including early stages. Br J Haematol 1998; 100: 15–23.
Thiele J, Kvasnicka HM, Zankovich R, Diehl V . The value of bone marrow histology in differentiating between early stage Polycythemia vera and secondary (reactive) Polycythemias. Haematologica 2001; 86: 368–374.
Pearson TC . Apparent polycythaemia. Blood Rev 1991; 5: 205–213.
Lamy T, Devillers A, Bernard M, Moisan A, Grulois I, Drenou B et al. Inapparent polycythemia vera—an unrecognized diagnosis. Am J Med 1997; 102: 14–20.
Berlin NI . Diagnosis and classification of the polycythemias. Sem Hematol 1975; 12: 339–351.
Fairbanks VF . Myeloproliferative disease: polycythemia vera: the packed cell volume and the curious logic of the red cell mass. Hematology 2000; 4: 381–395.
Sirhan S, Fairbanks VF, Tefferi A . Red cell mass and plasma volume measurements in polycythemia. Cancer 2005; 104: 213–215.
Thiele J, Kvasnicka HM, Vardiman J . Bone marrow histopathology in the diagnosis of chronic myeloproliferative disorders: a forgotten pearl. Best Pract Res Clin Haematol 2006; 19: 413–437.
Tefferi A, Pardanani A . Evaluation of ‘increased’ hemoglobin in the JAK2 mutations era: a diagnostic algorithm based on genetic tests. Mayo Clin Proc 2007; 82: 599–604.
Lippert E, Boissinot M, Kralovics R, Girodon F, Dobo I, Praloran V et al. The JAK2-V617F mutation is frequently present at diagnosis in patients with essential thrombocythemia and polycythemia vera. Blood 2006; 108: 1865–1867.
Tefferi A, Gilliland DG . The JAK2V617F tyrosine kinase mutation in myeloproliferative disorders: status report and immediate implications for disease classification and diagnosis. Mayo Clin Proc 2005; 80: 947–958.
Gattenlohner S, Peter C, Bonengel M, Einsele H, Bargou R, Muller-Hermelink HK et al. Detecting the JAK2 V617F mutation in fresh and ‘historic’ blood and bone marrow. Leukemia 2007; 21: 1559–1602.
Hermouet S, Dobo I, Lippert E, Boursier MC, Ergand L, Perrault-Hu F et al. Comparison of whole blood vs purified blood granulocytes for the detection and quantitation of JAK2(V617F). Leukemia 2007; 21: 1128–1130.
James C, Delhommeau F, Marzac C, Teyssandier I, Couedic JP, Giraudier S et al. Detection of JAK2 V617F as a first intention diagnostic test for erythrocytosis. Leukemia 2006; 20: 350–353.
McClure R, Mai M, Lasho T . Validation of two clinically useful assays for evaluation of JAK2 V617F mutation in chronic myeloproliferative disorders. Leukemia 2006; 20: 168–171.
Tefferi A . JAK2 mutations in polycythemia vera—molecular mechanisms and clinical applications. N Engl J Med 2007; 356: 444–445.
Remacha AF, Montserrat I, Santamaria A, Oliver A, Barcelo MJ, Parellada M . Serum erythropoietin in the diagnosis of polycythemia vera—a follow-up study. Haematologica 1997; 82: 406–410.
Messinezy M, Westwood NB, El-Hemaidi I, Marsden JT, Sherwood RS, Pearson TC . Serum erythropoietin values in erythrocytoses and in primary thrombocythaemia. Br J Haematol 2002; 117: 47–53.
Mossuz P, Girodon F, Donnard M, Latger-Cannard V, Dobo I, Boiret N et al. Diagnostic value of serum erythropoietin level in patients with absolute erythrocytosis. Haematologica 2004; 89: 1194–1198.
Thiele J, Kvasnicka HM . Hematopathologic findings in chronic idiopathic myelofibrosis. Semin Oncol 2005; 32: 380–394.
Di Nisio M, Barbui T, Di Gennaro L, Borrelli G, Finazzi G, Landolfi R et al. The haematocrit and platelet target in polycythemia vera. Br J Haematol 2007; 136: 249–259.
Michiels JJ, Berneman Z, Schroyens W, Kutti J, Swolin B, Ridell B et al. Philadelphia (Ph) chromosome-positive thrombocythemia without features of chronic myeloid leukemia in peripheral blood: natural history and diagnostic differentiation from Ph-negative essential thrombocythemia. Ann Hematol 2004; 83: 504–512.
Martiat P, Ifrah N, Rassool F, Morgan G, Giles F, Gow J et al. Molecular analysis of Philadelphia positive essential thrombocythemia. Leukemia 1989; 3: 563–565.
Lorand-Metze I, Vassallo J, Souza CA . Histological and cytological heterogeneity of bone marrow in Philadelphia-positive chronic myelogenous leukaemia at diagnosis. Br J Haematol 1987; 67: 45–49.
Elliott MA, Hanson CA, Dewald GW, Smoley SA, Lasho TL, Tefferi A . WHO-defined chronic neutrophilic leukemia: a long-term analysis of 12 cases and a critical review of the literature. Leukemia 2005; 19: 313–317.
Tefferi A, Pardanani A . Systemic mastocytosis: current concepts and treatment advances. Curr Hematol Rep 2004; 3: 197–202.
Tefferi A, Patnaik MM, Pardanani A . Eosinophilia: secondary, clonal and idiopathic. Br J Haematol 2006; 133: 468–492.
Bain B, Pierre R, Imbert M, Vardiman JW, Brunning RD, Flandrin G . Chronic eosinophilic leukemia and the hypereosinophilic syndrome. In: Jaffe ES, Harris NL, Stein H, Vardiman JW (eds) World Health Organization Classification of Tumors: Tumours of the Haematopoietic and Lymphoid Tissues. International Agency for Research on Cancer (IARC) Press: Lyon, France, 2001, 29–31.
Nowell PC, Hungerford DA . Chromosome studies on normal and leukemic human leukocytes. J Natl Cancer Inst 1960; 25: 85–109.
Groffen J, Stephenson JR, Heisterkamp N, de Klein A, Bartram CR, Grosveld G . Philadelphia chromosomal breakpoints are clustered within a limited region, bcr, on chromosome 22. Cell 1984; 36: 93–99.
Kralovics R, Passamonti F, Buser AS, Soon-Siong T, Tiedt R, Passweg JR et al. A gain of function mutation in Jak2 is frequently found in patients with myeloproliferative disorders. N Engl J Med 2005; 352: 1779–1790.
Nagata H, Worobec AS, Oh CK, Chowdhury BA, Tannenbaum S, Suzuki Y et al. Identification of a point mutation in the catalytic domain of the protooncogene c-kit in peripheral blood mononuclear cells of patients who have mastocytosis with an associated hematologic disorder. Proc Natl Acad Sci USA 1995; 92: 10560–10564.
Loh ML, Vattikuti S, Schubbert S, Reynolds MG, Carlson E, Lieuw KH et al. Mutations in PTPN11 implicate the SHP-2 phosphatase in leukemogenesis. Blood 2004; 103: 2325–2331.
Shannon KM, O'Connell P, Martin GA, Paderanga D, Olson K, Dinndorf P et al. Loss of the normal NF1 allele from the bone marrow of children with type 1 neurofibromatosis and malignant myeloid disorders. N Engl J Med 1994; 330: 597–601.
Lauchle JO, Braun BS, Loh ML, Shannon K . Inherited predispositions and hyperactive Ras in myeloid leukemogenesis. Pediatr Blood Cancer 2006; 46: 579–585.
Pardanani A, Hood J, Lasho T, Levine RL, Martin MB, Noronha G et al. TG101209, a small molecule JAK2-selective kinase inhibitor potently inhibits myeloproliferative disorder-associated JAK2V617F and MPLW515L/K mutations. Leukemia 2007; 21: 1658–1668.
Tefferi A, Vardiman JW . The diagnostic interface between histology and molecular tests in myeloproliferative disorders. Curr Opin Hematol 2007; 14: 115–122.
Boissinot M, Lippert E, Girodon F, Dobo I, Fouassier M, Masliah C et al. Latent myeloproliferative disorder revealed by the JAK2-V617F mutation and endogenous megakaryocytic colonies in patients with splanchnic vein thrombosis. Blood 2006; 108: 3223–3224.
Pardanani A, Lasho TL, Schwager S, Finke C, Hussein K, Pruthi RK et al. JAK2V617F prevalence and allele burden in non-splanchnic venous thrombosis in the absence of overt myeloproliferative disorder. Leukemia 2007; 21: 1828–1829.
Pardanani A, Lasho TL, Morice WG, Pruthi RK, Tefferi A . JAK2V617F is infrequently associated with arterial stroke in the absence of overt myeloproliferative disorder. J Thromb Haemost 2007; 5: 1784–1785.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tefferi, A., Vardiman, J. Classification and diagnosis of myeloproliferative neoplasms: The 2008 World Health Organization criteria and point-of-care diagnostic algorithms. Leukemia 22, 14–22 (2008). https://doi.org/10.1038/sj.leu.2404955
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.leu.2404955
Keywords
This article is cited by
-
Human gene-engineered calreticulin mutant stem cells recapitulate MPN hallmarks and identify targetable vulnerabilities
Leukemia (2023)
-
Essential Thrombocythemia in Adolescents and Young Adults: Clinical Aspects, Treatment Options and Unmet Medical Needs
Current Treatment Options in Oncology (2023)
-
Management of classical Philadelphia chromosome-negative myeloproliferative neoplasms in Asia: consensus of the Asian Myeloid Working Group
Clinical and Experimental Medicine (2023)
-
IRF4 and IRF8 expression are associated with clinical phenotype and clinico-hematological response to hydroxyurea in essential thrombocythemia
Frontiers of Medicine (2022)
-
Efficacy and safety of ropeginterferon alfa-2b in Japanese patients with polycythemia vera: an open-label, single-arm, phase 2 study
International Journal of Hematology (2022)