Abstract
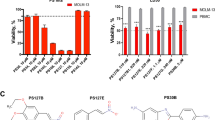
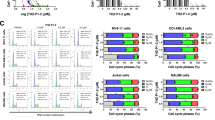
Aplidin (plitidepsin) is a novel marine-derived antitumor agent presently undergoing phase II clinical trials in hematological malignancies and solid tumors. Lack of bone marrow toxicity has encouraged further development of this drug for treatment of leukemia and lymphoma. Multiple signaling pathways have been shown to be involved in Aplidin-induced apoptosis and cell cycle arrest in G1 and G2 phase. However, the exact mechanism(s) of Aplidin action remains to be elucidated. Here we demonstrate that mitochondria-associated or -localized processes are the potential cellular targets of Aplidin. Whole genome gene-expression profiling (GEP) revealed that fatty acid metabolism, sterol biosynthesis and energy metabolism, including the tricarboxylic acid cycle and ATP synthesis are affected by Aplidin treatment. Moreover, mutant MOLT-4, human leukemia cells lacking functional mitochondria, were found to be resistant to Aplidin. Cytosine arabinoside (araC), which also generates oxidative stress but does not affect the ATP pool, showed synergism with Aplidin in our leukemia and lymphoma models in vitro and in vivo. These studies provide new insights into the mechanism of action of Aplidin. The efficacy of the combination of Aplidin and araC is currently being evaluated in clinical phase I/II program for the treatment of patients with relapsed leukemia and high-grade lymphoma.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Accession codes
References
Faivre S, Chieze S, Delbaldo C, Ady-Vago N, Guzman C, Lopez-Lazaro L et al. Phase I and pharmacokinetic study of Aplidine, a new marine cyclodepsipeptide in patients with advanced malignancies. J Clin Oncol 2005; 23: 7871–7880.
Jimeno J, Lopez-Martin JA, Ruiz-Casado A, Izquierdo MA, Scheuer PJ, Rinehart K . Progress in the clinical development of new marine-derived anticancer compounds. Anticancer Drugs 2004; 15: 321–329.
Mateos MV, Blade J, Prosper F, Lahuerta JJ, Garcia-Sanz R, Cibeira M et al. Preliminary report from an exploratory phase II trial with plitidepsin (Aplidin®) in patients with refractory/relapsed multiple myeloma. Blood 2005; 106: 2569 (abstracts).
Gomez SG, Bueren JA, Faircloth GT, Jimeno J, Albella B . In vitro toxicity of three new antitumoral drugs (trabectedin, Aplidin, and kahalalide F) on hematopoietic progenitors and stem cells. Exp Hematol 2003; 31: 1104–1111.
Gajate C, An F, Mollinedo F . Rapid and selective apoptosis in human leukemic cells induced by Aplidine through a Fas/CD95- and mitochondrial-mediated mechanism. Clin Cancer Res 2003; 9: 1535–1545.
Erba E, Serafini M, Gaipa G, Tognon G, Marchini S, Celli N et al. Effect of Aplidin in acute lymphoblastic leukaemia cells. Br J Cancer 2003; 89: 763–773.
Gajate C, Mollinedo F . Cytoskeleton-mediated death receptor and ligand concentration in lipid rafts forms apoptosis-promoting clusters in cancer chemotherapy. J Biol Chem 2005; 280: 11641–11647.
Garcia-Fernandez LF, Losada A, Alcaide V, Alvarez AM, Cuadrado A, Gonzalez L et al. Aplidin induces the mitochondrial apoptotic pathway via oxidative stress-mediated JNK and p38 activation and protein kinase C delta. Oncogene 2002; 21: 7533–7544.
Cuadrado A, Garcia-Fernandez LF, Gonzalez L, Suarez Y, Losada A, Alcaide V et al. Aplidin induces apoptosis in human cancer cells via glutathione depletion and sustained activation of the epidermal growth factor receptor, Src, JNK, and p38 MAPK. J Biol Chem 2003; 278: 241–250.
Cuadrado A, Gonzalez L, Suarez Y, Martinez T, Munoz A . JNK activation is critical for Aplidin-induced apoptosis. Oncogene 2004; 23: 4673–4680.
Taddei ML, Chiarugi P, Cuevas C, Ramponi G, Raugei G . Oxidation and inactivation of low molecular weight protein tyrosine phosphatase by the anticancer drug Aplidin. Int J Cancer 2006; 118: 2082–2088.
Gonzalez-Santiago L, Suarez Y, Zarich N, Munoz-Alonso MJ, Cuadrado A, Martinez T et al. Aplidin® induces JNK-dependent apoptosis in human breast cancer cells via alteration of glutathione homeostasis, Rac1 GTPase activation, and MKP-1 phosphatase downregulation. Cell Death Differ 2006; 13: 1968–1981.
Losada A, Lopez-Oliva JM, Sanchez-Puelles JM, Garcia-Fernandez LF . Establishment and characterisation of a human carcinoma cell line with acquired resistance to Aplidin. Br J Cancer 2004; 91: 1405–1413.
Bresters D, Broekhuizen AJ, Kaaijk P, Faircloth GT, Jimeno J, Kaspers GJ . In vitro cytotoxicity of Aplidin and crossresistance with other cytotoxic drugs in childhood leukemic and normal bone marrow and blood samples: a rational basis for clinical development. Leukemia 2003; 17: 1338–1343.
Armand R, Channon JY, Kintner J, White KA, Miselis KA, Perez RP et al. The effects of ethidium bromide induced loss of mitochondrial DNA on mitochondrial phenotype and ultrastructure in a human leukemia T-cell line (MOLT-4 cells). Toxicol Appl Pharmacol 2004; 196: 68–79.
Chou TC, Talalay P . Quantitative analysis of dose–effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul 1984; 22: 27–55.
Darzynkiewicz Z, Li X, Bedner E . Use of flow and laser-scanning cytometry in analysis of cell death. Methods Cell Biol 2001; 66: 69–109.
Dennis Jr G, Sherman BT, Hosack DA, Yang J, Gao W, Lane HC et al. DAVID: Database for Annotation, Visualization, and Integrated Discovery. Genome Biol 2003; 4: P3.
Doniger SW, Salomonis N, Dahlquist KD, Vranizan K, Lawlor SC, Conklin BR . MAPPFinder: using gene ontology and GenMAPP to create a global gene-expression profile from microarray data. Genome Biol 2003; 4: R7.
Goto Y, Matsuzaki Y, Kurihara S, Shimizu A, Okada T, Yamamoto K et al. A new melanoma antigen fatty acid-binding protein 7, involved in proliferation and invasion, is a potential target for immunotherapy and molecular target therapy. Cancer Res 2006; 66: 4443–4449.
Majima HJ, Oberley TD, Furukawa K, Mattson MP, Yen HC, Szweda LI et al. Prevention of mitochondrial injury by manganese superoxide dismutase reveals a primary mechanism for alkaline-induced cell death. J Biol Chem 1998; 273: 8217–8224.
Geller HM, Cheng KY, Goldsmith NK, Romero AA, Zhang AL, Morris EJ et al. Oxidative stress mediates neuronal DNA damage and apoptosis in response to cytosine arabinoside. J Neurochem 2001; 78: 265–275.
Gunter TE, Yule DI, Gunter KK, Eliseev RA, Salter JD . Calcium and mitochondria. FEBS Lett 2004; 567: 96–102.
O'Reilly CM, Fogarty KE, Drummond RM, Tuft RA, Walsh Jr JV . Quantitative analysis of spontaneous mitochondrial depolarizations. Biophys J 2003; 85: 3350–3357.
Modica-Napolitano JS, Singh K . Mitochondria as targets for detection and treatment of cancer. Expert Rev Mol Med 2002, 1–19.
Acknowledgements
This work was supported by a grant from PharmaMar, S.A. to DB.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Leukemia website (http://www.nature.com/leu)
Rights and permissions
About this article
Cite this article
Humeniuk, R., Menon, L., Mishra, P. et al. Aplidin synergizes with cytosine arabinoside: functional relevance of mitochondria in Aplidin-induced cytotoxicity. Leukemia 21, 2399–2405 (2007). https://doi.org/10.1038/sj.leu.2404911
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.leu.2404911