Abstract
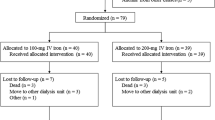
This randomized study assessed if intravenous iron improves hemoglobin (Hb) response and permits decreased epoetin dose in anemic (Hb 9–11 g/dl), transfusion-independent patients with stainable iron in the bone marrow and lymphoproliferative malignancies not receiving chemotherapy. Patients (n=67) were randomized to subcutaneous epoetin beta 30 000 IU once weekly for 16 weeks with or without concomitant intravenous iron supplementation. There was a significantly (P<0.05) greater increase in mean Hb from week 8 onwards in the iron group and the percentage of patients with Hb increase ⩾2 g/dl was significantly higher in the iron group (93%) than in the no-iron group (53%) (per-protocol population; P=0.001). Higher serum ferritin and transferrin saturation in the iron group indicated that iron availability accounted for the Hb response difference. The mean weekly patient epoetin dose was significantly lower after 13 weeks of therapy (P=0.029) and after 15 weeks approximately 10 000 IU (>25%) lower in the iron group, as was the total epoetin dose (P=0.051). In conclusion, the Hb increase and response rate were significantly greater with the addition of intravenous iron to epoetin treatment in iron-replete patients and a lower dose of epoetin was required.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Rizzo JD, Lichtin AE, Woolf SH, Seidenfeild J, Bennett CL, Cella D et al. Use of epoetin in patients with cancer: evidence-based clinical practice guidelines of the American Society of Clinical Oncology and the American Society of Hematology. Blood 2002; 100: 2303–2320.
Bokemeyer C, Aapro MS, Courdi A, Foubent J, Link H, Österborg A . EORTC guidelines for the use of erythropoietic proteins in anaemic patients with cancer. Eur J Cancer 2004; 40: 2201–2216.
National Comprehensive Cancer Network. Clinical Practice Guidelines in Oncology – v.2.2006. Cancer- and treatment-related anemia. Version 2.2006. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp. Accessed April 19, 2006.
Roy CN, Andrews NC . Anemia of inflammation: the hepcidin link. Curr Opin Hematol 2005; 12: 107–111.
Nemeth E, Ganz T . Regulation of iron metabolism by hepcidin. Annu Rev Nutr 2006; 26: 323–342.
Glaspy J, Cavill I . Role of iron in optimizing responses of anemic cancer patients to erythropoietin. Oncology 1999; 13: 461–473.
Weiss G, Goodnough LT . Anemia of chronic disease. N Engl J Med 2005; 352: 1011–1023.
Glaspy J, Beguin Y . Anaemia management strategies: optimising treatment using epoetin beta (NeoRecormon). Oncology 2005; 69 (Suppl 2): 8–16.
Cavill I, Auerbach M, Bailie GR, Barrett-Lee P, Beguin Y, Kaltwasser P et al. Iron and the anaemia of chronic disease: a review and strategic recommendations. Curr Med Res Opin 2006; 22: 731–737.
Macdougall IC . Strategies for iron supplementation: oral versus intravenous. Kidney Int 1999; 69 (Suppl): S61–S66.
Locatelli F, Aljama P, Barany P, Canaud B, Carera F, Eckardt KU et al. European Best Practice Guidelines Working Group. Revised European best practice guidelines for the management of anaemia in patients with chronic renal failure. Nephrol Dial Transplant 2004; 19 (Suppl 2): ii1–ii47.
Auerbach M, Ballard H, Trout RJ, McIlwain M, Ackerman A, Bahrain H et al. Intravenous iron optimizes the response to recombinant human erythropoietin in cancer patients with chemotherapy-related anemia: a multicenter, open-label, randomized trial. J Clin Oncol 2004; 22: 1301–1307.
Henry DH . The role of intravenous iron in cancer-related anemia. Oncology (Williston Park) 2006; 20 (Suppl 6): 21–24.
Junca J, Fernandez-Aviles F, Oriol A, Navarro JT, Milla F, Sancho JM et al. The usefulness of the serum transferrin receptor in detecting iron deficiency in the anemia of chronic disorders. Haematologica 1998; 83: 676–680.
Mast AE, Blinder MA, Lu Q, Flax S, Dietzen DJ . Clinical utility of the reticulocyte hemoglobin content in the diagnosis of iron deficiency. Blood 2002; 99: 1489–1491.
Cazzola M, Beguin Y, Kloczko J, Spicka I, Coiffier B . Once-weekly epoetin beta is highly effective in treating anaemic patients with lymphoproliferative malignancy and defective endogenous erythropoietin production. Br J Haematol 2003; 122: 386–393.
Österborg A, Brandberg Y, Molotova V, Iosava G, Abdulkadyrov K, Hedenus M et al. Randomized, double-blind, placebo-controlled trial of recombinant human erythropoietin, epoetin beta, in hematologic malignancies. J Clin Oncol 2002; 20: 2486–2494.
Hedenus M, Adriansson M, San Miguel J, Kramer MH, Schipperus MR, Juvonen E et al. Efficacy and safety of darbepoetin alfa in anaemic patients with lymphoproliferative malignancies: a randomized, double-blind, placebo-controlled study. Br J Haematol 2003; 122: 394–403.
Dammacco F, Castoldi G, Rödjer S . Efficacy of epoetin alfa in the treatment of anemia of multiple myeloma. Br J Haematol 2001; 113: 172–179.
Besarab A, Frinak S, Yee J . An indistinct balance: the safety and efficacy of parenteral iron therapy. J Am Soc Nephrol 1999; 10: 2029–2043.
Bohlius J, Weingart O, Trelle S, Engert A . Cancer-related anemia and recombinant human erythropoietin – an updated overview. Nat Clin Pract Oncol 2006; 3: 152–164.
Acknowledgements
We thank the patients and study nurses for their dedication to this trial. We thank Karin Larsson for monitoring and assisting and Ulf Jansson for excellent laboratory work. We also thank the NIFe Study Group for its participation in this study; a complete membership list appears in ‘ Appendix.’ This work was supported by grants from Roche AB, Sweden, and the Research and Development Centre, Sundsvall Hospital, Sundsvall, Sweden. This investigator-initiated study was supported in part by research funding from Roche AB, Sweden.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
The NIFe study group included the following individuals and centers
Jesper Aagesen, Department of Medicine, Jönköping Hospital; Lucia Ahlberg, Department of Medicine, University Hospital, Linköping; Gunnar Birgegård, Department of Hematology, Akademiska Hospital; Margareta Carlsson, Department of Medicine, Växjö Hospital; Leif Enquist, Department of Medicine, Värnamo Hospital; Torbjörn Karlsson, Department of Medicine, St Görans Hospital; Sören Hanssen, Department of Medicine, Högland Hospital; Michael Hedenus, Department of Medicine, Sundsvall Hospital; Gerd Lärfars, Department of Medicine, Södersjukhuset; Birgitta Lauri, Department of Medicine, Sunderby Hospital; Olof Lindquist, Department of Medicine, Uddevalla Hospital; Jeanette Lundin, Departments of Hematology and Oncology, Karolinska University Hospital; Per Näsman, Center for Safety Research, Royal Institute of Technology; Göran Nilsson, Department of Medicine, Östra Hospital; Herman Nilsson-Ehle, Medical Department, Sahlgrenska University Hospital; Anders Österborg, Departments of Hematology and Oncology, Karolinska University Hospital; Fredrik Sjöö, Department of Medicine, St Görans Hospital, and Kristina Wallman, Medical Department, Falun Hospital.
Rights and permissions
About this article
Cite this article
Hedenus, M., Birgegård, G., Näsman, P. et al. Addition of intravenous iron to epoetin beta increases hemoglobin response and decreases epoetin dose requirement in anemic patients with lymphoproliferative malignancies: a randomized multicenter study. Leukemia 21, 627–632 (2007). https://doi.org/10.1038/sj.leu.2404562
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.leu.2404562
Keywords
This article is cited by
-
Randomized trial of sucrosomial iron supplementation in patients with chemotherapy-related anemia treated with ESA
Supportive Care in Cancer (2022)
-
Iron Sucrose: A Wealth of Experience in Treating Iron Deficiency
Advances in Therapy (2020)
-
Use of iron sucrose and red blood cell transfusions in anaemic cancer patients in France (OncoFer study)
Supportive Care in Cancer (2017)
-
Management of anemia and iron deficiency in a cancer center in France
Supportive Care in Cancer (2016)
-
Iron metabolism and iron supplementation in cancer patients
Wiener klinische Wochenschrift (2015)