Abstract
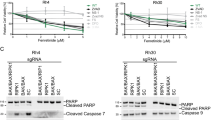
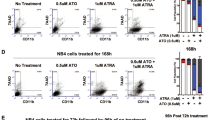
Among the topoisomerase (topo) II isozymes (α and β), topo IIβ has been suggested to regulate differentiation. In this study, we examined the role of topo IIβ in all-trans retinoic acid (ATRA)-induced differentiation of myeloid leukemia cell lines. Inhibition of topo IIβ activity or downregulation of protein expression enhanced ATRA-induced differentiation/growth arrest and apoptosis. ATRA-induced apoptosis in topo IIβ-deficient cells involved activation of the caspase cascade and was rescued by ectopic expression of topo IIβ. Gene expression profiling led to the identification of peroxiredoxin 2 (PRDX2) as a candidate gene that was downregulated in topo IIβ-deficient cells. Reduced expression of PRDX2 validated at the mRNA and protein level, in topo IIβ-deficient cells correlated with increased accumulation of reactive oxygen species (ROS) following ATRA-induced differentiation. Overexpression of PRDX2 in topo IIβ-deficient cells led to reduced accumulation of ROS and partially reversed ATRA-induced apoptosis. These results support a role for topo IIβ in survival of ATRA-differentiated myeloid leukemia cells. Reduced expression of topo IIβ induces apoptosis in part by impairing the anti-oxidant capacity of the cell owing to downregulation of PRDX2. Thus, suppression of topo IIβ and/or PRDX2 levels in myeloid leukemia cells provides a novel approach for improving ATRA-based differentiation therapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Kellner U, Sehested M, Jensen PB, Giesler F, Rudolph P . Culprit and victim – DNA topoisomerase II. Lancet Oncol 2002; 3: 235–243.
Li T-K, Liu LF . Tumor cell death induced by topoisomerase-targeting drugs. Annu Rev Pharmacol 2001; 41: 53–57.
Wang JC . Cellular roles of DNA topoisomerases: a molecular perspective. Nat Rev Mol Cell Biol 2002; 3: 430–440.
Turley H, Comley M, Houlbrook S, Nozaki N, Kikuchi A, Hickson ID et al. The distribution and expression of the two isoforms of DNA topoisomerase II in normal and neoplastic human tissues. Br J Cancer 1997; 75: 1340–1346.
Capranico G, Tinelli S, Austin CA, Fisher ML, Zunino F . Different patterns of gene expression of topoisomerase II isoforms in differentiated tissues durine murine development. Biochim Biophys Acta 1992; 1132: 43–48.
Woessner RD, Mattern MR, Mirabelli CK, Johnson RK, Drake FH . Proliferation- and cell cycle-dependent differences in expression of topoisomerase II in NIH-3T3 cells. Cell Growth Differ 1991; 2: 209–214.
Yang X, Li W, Prescott ED, Burden SJ, Wang JC . DNA topoisomerase IIβ in neuronal development. Science 2000; 287: 131–134.
Tsutsui K, Tsutsui K, Sano K, Kikuchi A, Tokunaga A . Involvement of DNA topoisomerase IIβ in neuronal differentiation. J Biol Chem 2001; 276: 5769–5778.
Lyu YL, Wang JC . Aberrant lamination in the cerebral cortex of mouse embryos lacking DNA topoisomerase IIβ. Proc Natl Acad Sci USA 2003; 100: 7123–7128.
Kaufmann SH, McLaughlin SJ, Kastan MB, Liu LF, Karp JE, Burke PJ . Topoisomerase II levels during granulocytic maturation in vitro and in vivo. Cancer Res 1991; 51: 3534–3543.
Kaufmann SH, Charron M, Burke PJ, Karp JE . Changes in topoisomerase I levels and localization during myeloid maturation in vitro and in vivo. Cancer Res 1995; 55: 1255–1260.
Aoyama M, Grabowski DR, Isaacs RJ, Krivacic KA, Rybicki LA, Bukowski RM et al. Altered expression and activity of topoisomerases during all trans retinoic acid induced differentiation of HL-60 cells. Blood 1998; 92: 2863–2870.
Chresta CM, Hall BF, Francis GF . Retinoic acid and phorbol ester induced hyperphosphorylation of topoisomerase II is an early event in HL-60 human leukemia cell differentiation: effect on topoisomerase activity and etoposide sensitivity. Leukemia 1995; 9: 1373–1381.
Kim HJ, Lotan R . Identification of retinoid-modulated proteins in squamous carcinoma cells using high-throughput immunoblotting. Cancer Res 2004; 64: 2439–2448.
Noy N . Retinoid-binding proteins: mediators of retinoid action. Biochem J 2000; 348: 195–481.
Melnick A, Licht JD . Deconstructing a disease: RAR α, its fusion partners, and their roles in the pathogenesis of acute promyelocytic leukemia. Blood 1999; 93: 3167–3215.
Collins SJ . The role of retinoid and retinoic acid receptors in normal hematopoiesis. Leukemia 2002; 16: 1896–1905.
Bastien J, Rochette-Egly C . Nuclear retinoid receptors and the transcription of retinoid-target genes. Gene 2004; 328: 1–16.
Kastner P, Chan S . Function of RARα during the maturation of neutrophils. Oncogene 2001; 20: 7178–7185.
Altucci L, Gronemeyer H . The promise of retinoids to fight against cancer. Nat Rev Cancer 2001; 1: 181–193.
Zhang P, Liu B, Kang SW, Seo MS, Rhee SG, Obeid LM . Thioredoxin peroxidase is a novel inhibitor of apoptosis with a mechanism distinct from that of bcl-2. J Biol Chem 1997; 272: 30615–30639.
Herzog CE, Holmes KA, Tuschong LM, Ganapathi R, Zwelling LA . Absence of topoisomerase IIβ in an amsacrine-resistant human leukemia cell line with mutant topoisomerase IIα. Cancer Res 1998; 58: 5298–5300.
Sun Y, Kim SH, Zhou D-C, Ding W, Paietta E, Guidez F et al. Acute promyelocytic leukemia cell line AP-1060 establishes as a cytokine-dependent culture from a patient clinically resistant to all-trans-retinoic acid and arsenic trioxide. Leukemia 2004; 18: 1258–1269.
Brummelkamp TR, Bernards R, Agami R . A system for stable expression of short interfering RNAs in mammalian cells. Science 2002; 296: 550–553.
Gurova KV, Hill JE, Razorenova OV, Chumakov PM, Gudkov AV . p53 pathway in renal cell carcinoma is repressed by a dominant mechanism. Cancer Res 2004; 64: 1951–1958.
Yen A, Varvayanis S, Smith JL, Lamkin TJ . Retinoic acid induces expression of SLP-76: expression with c-FMS enhances ERK activation and retinoic-acid induced differentiation/G0 arrest of HL-60 cells. Eur J Cell Biol 2006; 85: 117–132.
Tabata M, Tabata R, Grabowski DR, Bukowski RM, Ganapathi MK, Ganapathi R . Roles of NF-κB and 26S proteasome in apoptotic cell death induced by topoisomerase I and II poisons in human non-small cell lung carcinoma. J Biol Chem 2001; 276: 8029–8036.
Grabowski DR, Holmes KA, Aoyama M, Ye Y, Rybicki LA, Bukowski RM et al. Altered drug interaction and regulation of topoisomerase IIβ: potential mechanisms governing sensitivity of HL-60 cells to amsacrine and etoposide. Mol Pharmacol 1999; 56: 1340–1345.
Huang P, Feng L, Oldham EA, Keating MJ, Plunkett W . Superoxide dismutase as a target for the selective killing of cancer cells. Nature 2000; 407: 390–395.
Kwon J, Lee SR, Yang KS, Ahn Y, Kim YJ, Stadtman ER et al. Reversible oxidation inactivation of the tumor suppressor PTEN in cells stimulated with peptide growth factors. Proc Natl Acad Sci USA 2004; 101: 16419–16424.
Acknowledgements
This work was supported by USPHS Grants RO1 CA74939, RO1 DK56917 and RO1 CA117928. Eli Zarkhin was supported by a Joseph S Silber Student Fellowship, American Cancer Society, Cuyahoga County Unit. We thank Dr R Gallagher, Albert Einstein College of Medicine, Bronx, New York for generously providing the AP-1060 cells; Dr J Maciejewski, Cleveland Clinic Foundation, Cleveland, Ohio for generously providing the KG1 cells; and Dr I Hickson for the generous gift of topo IIα antibody. We gratefully acknowledge Terri O'Brian and Jim Reed of the Art-Medical Illustrations and Photography department for skillful preparation of the artwork.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Leukemia website (http://www.nature.com/leu)
Rights and permissions
About this article
Cite this article
Chikamori, K., Hill, J., Grabowski, D. et al. Downregulation of topoisomerase IIβ in myeloid leukemia cell lines leads to activation of apoptosis following all-trans retinoic acid-induced differentiation/growth arrest. Leukemia 20, 1809–1818 (2006). https://doi.org/10.1038/sj.leu.2404351
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.leu.2404351
Keywords
This article is cited by
-
Topoisomerase II β Gene Specific siRNA Delivery by Nanoparticles Prepared with c-ter Apotransferrin and its Effect on HIV-1 Replication
Molecular Biotechnology (2021)
-
Role of non-coding RNA networks in leukemia progression, metastasis and drug resistance
Molecular Cancer (2020)
-
Association of immunophenotype with expression of topoisomerase II α and β in adult acute myeloid leukemia
Scientific Reports (2020)
-
Dose-Dependent Reactive Species Accumulation and Preferential Double-Strand Breaks Repair are Featured in the γ-ray Response in Medicago truncatula Cells
Plant Molecular Biology Reporter (2014)
-
Distinct gene signature revealed in white blood cells, CD4+ and CD8+ T cells in (NZBx NZW) F1 lupus mice after tolerization with anti-DNA Ig peptide
Genes & Immunity (2010)