Abstract
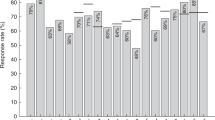
Intensified chemotherapy regimens resulting in improved survival of children with acute lymphocytic leukemia (ALL) lead to concerns about therapy-induced immune damage reflected by the loss of protection of previous immunizations and the efficacy of (re-)vaccination. The severity of secondary immunodeficiency, however, is not clear and knowledge is based on a limited number of studies. We performed a systematic review on literature concerning vaccination data of children with ALL published since 1980. Eight studies fulfilled the inclusion criteria. Regarding antibody titers after treatment, the number of children who had preserved the defined protection level for antibodies differed widely, ranging from 17 to 98% for diphtheria, 27 to 82% for Bordetella pertussis, 20 to 98% for tetanus, 62 to 100% for poliomyelitis, 35 to 100% for Haemophilus influenzae type B (HiB), 29 to 92% for mumps, 29 to 60% for measles and 72 to 92% for rubella. Most patients however responded to revaccination, demonstrating immunological recovery. Although the designs and results of the included studies varied widely, it can be concluded that cytostatic therapy for ALL in children results in a temporarily reduction of specific antibody levels. Memory is preserved but revaccination may be warranted. This is the first systematic review and the best possible current approximation of chemotherapy-induced immune damage in children after ALL treatment.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Carroll WL, Bhojwani D, Min DJ, Raetz E, Relling M, Davies S et al. Pediatric acute lymphoblastic leukemia. Hematology (Am Soc Hematol Educ Program) 2003; 1: 102–131.
Pui CH, Sandlund JT, Pei D, Campana D, Rivera GK, Ribeiro RC et al. Improved outcome for children with acute lymphoblastic leukemia: results of Total Therapy Study XIIIB at St Jude Children's Research Hospital. Blood 2004; 104: 2690–2696.
Pui CH, Relling MV, Downing JR . Acute lymphoblastic leukemia. N Engl J Med 2004; 350: 1535–1548.
Slats AM, Egeler RM, van der Does van den B, Korbijn C, Hahlen K, Kamps WA et al. Causes of death – other than progressive leukemia – in childhood acute lymphoblastic (ALL) and myeloid leukemia (AML): the Dutch Childhood Oncology Group experience. Leukemia 2005; 19: 537–544.
Pui CH, Evans WE . Treatment of acute lymphoblastic leukemia. N Engl J Med 2006; 354: 166–178.
Cesaro S . Which immunization for children after chemotherapy? Eur J Haematol 2005; 75: 174–180.
Ek T, Mellander L, Abrahamsson J . Re-immunisations after childhood leukaemia. Eur J Haematol 2005; 75: 175–176.
Fioredda F, Giacchino M, Castagnola E . Assessment of humoral immunity to poliomyelitis, tetanus, hepatitis B, measles, rubella, and mumps in children after chemotherapy. Cancer 2005; 103: 1758–1759.
Laws HJ, Gobel U, Calaminus G . Do we really know which pediatric cancer patients need revaccination? Eur J Haematol 2005; 75: 177–178.
Laws HJ, Calaminus G, Gobel U . Assessment of humoral immunity to poliomyelitis, tetanus, hepatitis B, measles, rubella, and mumps in children after chemotherapy. Cancer 2005; 103: 1759.
van Wering ER, van der Linden-Schrever BE, Szczepanski T, Willemse MJ, Baars EA, Wijngaarde-Schmitz HM et al. Regenerating normal B-cell precursors during and after treatment of acute lymphoblastic leukaemia: implications for monitoring of minimal residual disease. Br J Haematol 2000; 110: 139–146.
Alanko S, Pelliniemi TT, Salmi TT . Recovery of blood B-lymphocytes and serum immunoglobulins after chemotherapy for childhood acute lymphoblastic leukemia. Cancer 1992; 69: 1481–1486.
Mustafa MM, Buchanan GR, Winick NJ, McCracken GH, Tkaczewski I, Lipscomb M et al. Immune recovery in children with malignancy after cessation of chemotherapy. J Pediatr Hematol Oncol 1998; 20: 451–457.
Ek T, Mellander L, Andersson B, Abrahamsson J . Immune reconstitution after childhood acute lymphoblastic leukemia is most severely affected in the high risk group. Pediatr Blood Cancer 2004; 44: 461–468.
Haining WN, Neuberg DS, Keczkemethy HL, Evans JW, Rivoli S, Gelman R et al. Antigen-specific T-cell memory is preserved in children treated for acute lymphoblastic leukemia. Blood 2005; 106: 1749–1754.
Ek T, Mellander L, Hahn-Zoric M, Abrahamsson J . Intensive Treatment for Childhood Acute Lymphoblastic Leukemia Reduces Immune Responses to Diphtheria, Tetanus, and Haemophilus influenzae Type b. J Pediatr Hematol Oncol 2004; 26: 727–734.
Rothman KJ . Analyzing Simple Epidemiologic Data. Epidemiology, vol 1. Oxford University Press: New York, 2002, pp 130–143.
Wilson EB . Probable inference. The law of succession and statistical inference. J Am Stat Assoc 1927; 22: 209–212.
Nyerges G, Zimonyi I, Nyerges G, Meszner Z, Marosi A . Efficiency of tetanus toxoid booster in leukaemic children. Acta Paediatr Acad Sci Hung 1981; 22: 237–241.
Torigoe S, Hirai S, Oitani K, Ito M, Ihara T, Iwasa T et al. Application of live attenuated measles and mumps vaccines in children with acute leukemia. Biken J 1981; 24: 147–151.
Stenvik M, Hovi L, Siimes MA, Roivainen M, Hovi T . Antipolio prophylaxis of immunocompromised children during a nationwide oral poliovaccine campaign. Pediatr Infect Dis J 1987; 6: 1106–1110.
Martin II, Arce CA, Cruz MO, Estella AJ, Martin Mateos MA . Humoral immunity in pediatric patients with acute lymphoblastic leukaemia. Allergol Immunopathol (Madrid) 2003; 31: 303–310.
Hamarstrom V, Pauksen K, Svensson H, Oberg G, Paul C, Ljungman P . Tetanus immunity in patients with hematological malignancies. Support Care Cancer 1998; 6: 469–472.
Feldman S, Gigliotti F, Shenep JL, Roberson PK, Lott L . Risk of Haemophilus influenzae type b disease in children with cancer and response of immunocompromised leukemic children to a conjugate vaccine. J Infect Dis 1990; 161: 926–931.
Kaplan SL, Duckett T, Mahoney Jr DH, Kennedy LL, Dukes CM, Schaffer DM et al. Immunogenicity of Haemophilus influenzae type b polysaccharide–tetanus protein conjugate vaccine in children with sickle hemoglobinopathy or malignancies, and after systemic Haemophilus influenzae type b infection. J Pediatr 1992; 120: 367–370.
Kung FH, Orgel HA, Wallace WW, Hamburger RN . Antibody production following immunization with diphtheria and tetanus toxoids in children receiving chemotherapy during remission of malignant disease. Pediatrics 1984; 74: 86–89.
Lange B, Jakacki R, Nasab AH, Luery N, McVerry PH . Immunization of leukemic children with Haemophilus conjugate vaccine. Pediatr Infect Dis J 1989; 8: 883–884.
Rautonen J, Siimes MA, Lundstrom U, Pettay O, Lanning M, Salmi TT et al. Vaccination of children during treatment for leukemia. Acta Paediatr Scand 1986; 75: 579–585.
Ridgway D, Wolff LJ, Deforest A . Immunization response varies with intensity of acute lymphoblastic leukemia therapy. Am J Dis Child 1991; 145: 887–891.
Shenep JL, Feldman S, Gigliotti F, Roberson PK, Marina N, Foreschle JE et al. Response of immunocompromised children with solid tumors to a conjugate vaccine for Haemophilus influenzae type b. J Pediatr 1994; 125: 581–584.
Weisman SJ, Cates KL, Allegretta GJ, Quinn JJ, Altman AJ . Antibody response to immunization with Haemophilus influenzae type b polysaccharide vaccine in children with cancer. J Pediatr 1987; 111: 727–729.
Feldman S, Gigliotti F, Bockhold C, Naegele R . Measles and rubella antibody status in previously vaccinated children with cancer. Med Pediatr Oncol 1988; 16: 308–311.
Feldman S, Andrew M, Norris M, McIntyre B, Iyer R . Decline in rates of seropositivity for measles, mumps, and rubella antibodies among previously immunized children treated for acute leukemia. Clin Infect Dis 1998; 27: 388–390.
Kantar M, Cetingul N, Kansoy S, Kutukculer N, Aksu G . Immune deficiencies following cancer treatment in children. J Trop Pediatr 2003; 49: 286–290.
Reinhardt D, Houliara K, Pekrun A, Lakomek M, Krone B . Impact of conventional chemotherapy on levels of antibodies against vaccine-preventable diseases in children treated for cancer. Scand J Infect Dis 2003; 35: 851–857.
von der Hardt K, Jungert J, Beck JD, Heininger U . Humoral immunity against diphtheria, tetanus and poliomyelitis after antineoplastic therapy in children and adolescents – a retrospective analysis. Vaccine 2000; 18: 2999–3004.
Zignol M, Peracchi M, Tridello G, Pillon M, Fregonese F, D’Elia R et al. Assessment of humoral immunity to poliomyelitis, tetanus, hepatitis B, measles, rubella, and mumps in children after chemotherapy. Cancer 2004; 101: 635–641.
Schuller E, Forster-Waldl E, Slavc I, Maurer W . Immunity against vaccine-preventable potentially neurotropic diseases in children treated for malignant brain tumours with HIT-91 chemo- and radiotherapy. Eur J Cancer 2004; 40: 236–244.
Ercan TE, Soycan LY, Apak H, Celkan T, Ozkan A, Akdenizli E et al. Antibody titers and immune response to diphtheria–tetanus–pertussis and measles–mumps–rubella vaccination in children treated for acute lymphoblastic leukemia. J Pediatr Hematol Oncol 2005; 27: 273–277.
Smith S, Schiffman G, Karayalcin G, Bonagura V . Immunodeficiency in long-term survivors of acute lymphoblastic leukemia treated with Berlin–Frankfurt–Munster therapy. J Pediatr 1995; 127: 68–75.
Brodtman DH, Rosenthal DW, Redner A, Lanzkowsky P, Bonagura VR . Immunodeficiency in children with acute lymphoblastic leukemia after completion of modern aggressive chemotherapeutic regimens. J Pediatr 2005; 146: 654–661.
Lehrnbecher T, Foster C, Vazquez N, Mackall CL, Chanock SJ . Therapy-induced alterations in host defense in children receiving therapy for cancer. J Pediatr Hematol Oncol 1997; 19: 399–417.
Ridgway D, Wolff LJ . Active immunization of children with leukemia and other malignancies. Leukemia Lymphoma 1993; 9: 177–192.
Pirofski LA, Casadevall A . Use of licensed vaccines for active immunization of the immunocompromised host. Clin Microbiol Rev 1998; 11: 1–26.
Kyaw MH, Rose Jr CE, Fry AM, Singleton JA, Moore Z, Zell ER et al. The influence of chronic illnesses on the incidence of invasive pneumococcal disease in adults. J Infect Dis 2005; 192: 377–386.
Nilsson A, De Milito A, Engstrom P, Nordin M, Narita M, Grillner L et al. Current chemotherapy protocols for childhood acute lymphoblastic leukemia induce loss of humoral immunity to viral vaccination antigens. Pediatrics 2002; 109: e91.
van der Does-van den Berg A, Hermans J, Nagel J, van Steenis G . Immunity to diphtheria, pertussis, tetanus, and poliomyelitis in children with acute lymphocytic leukemia after cessation of chemotherapy. Pediatrics 1981; 67: 222–229.
Fioredda F, Plebani A, Hanau G, Haupt R, Giacchino M, Barisone E et al. Re-immunisation schedule in leukaemic children after intensive chemotherapy: a possible strategy. Eur J Haematol 2005; 74: 20–23.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests: none
Appendix A.
Appendix A.
Search strategy:
Antibody Formation OR Immunocompetence OR Diphtheria Toxoid OR Pertussis Vaccine OR Tetanus Toxoid OR Poliovirus Vaccines OR Haemophilus Vaccines OR Mumps Vaccine OR Measles Vaccine OR Rubella Vaccine OR humoral immunity* AND Neoplasms OR Cancer NOT Stem Cell Transplantation Limits.
All Child: 0–18 years, Publication Date from 1980, English, Humans.
*Please note that ‘humoral immunity’ is not a MESH term but was used as a text word.
Rights and permissions
About this article
Cite this article
van Tilburg, C., Sanders, E., Rovers, M. et al. Loss of antibodies and response to (re-)vaccination in children after treatment for acute lymphocytic leukemia: a systematic review. Leukemia 20, 1717–1722 (2006). https://doi.org/10.1038/sj.leu.2404326
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.leu.2404326
Keywords
This article is cited by
-
Antibody waning after immunosuppressive chemotherapy and immunomodulators, re-immunization considerations in pediatric patients with malignancy and chronic immune thrombocytopenic purpura
BMC Infectious Diseases (2022)
-
Molecular characterization of hepatitis B virus (HBV) isolated from a pediatric case of acute lymphoid leukemia, with a delayed response to antiviral treatment: a case report
BMC Pediatrics (2022)
-
Impfen bei Immundefizienz
Bundesgesundheitsblatt - Gesundheitsforschung - Gesundheitsschutz (2020)
-
Impfung bei Immunsuppression
Monatsschrift Kinderheilkunde (2013)
-
Impfungen bei onkologischen Patienten
best practice onkologie (2008)