Abstract
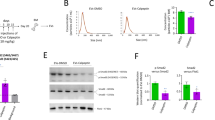
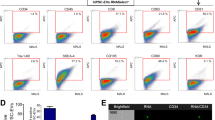
Membrane-derived vesicles (MV) are released from the surface of activated eucaryotic cells and exert pleiotropic effects on surrounding cells. Since the maintenance of pluripotency and undifferentiated propagation of embryonic stem (ES) cells in vitro requires tight cell to cell contacts and effective intercellular signaling, we hypothesize that MV derived from ES cells (ES-MV) express stem cell-specific molecules that may also support self-renewal and expansion of adult stem cells. To address this hypothesis, we employed expansion of hematopoietic progenitor cells (HPC) as a model. We found that ES-MV (10 μg/ml) isolated from murine ES cells (ES-D3) in serum-free cultures significantly (i) enhanced survival and improved expansion of murine HPC, (ii) upregulated the expression of early pluripotent (Oct-4, Nanog and Rex-1) and early hematopoietic stem cells (Scl, HoxB4 and GATA 2) markers in these cells, and (iii) induced phosphorylation of MAPK p42/44 and serine-threonine kinase AKT. Furthermore, molecular analysis revealed that ES-MV express Wnt-3 protein and are selectively highly enriched in mRNA for several pluripotent transcription factors as compared to parental ES cells. More important, this mRNA could be delivered by ES-MV to target cells and translated into the corresponding proteins. The biological effects of ES-MV were inhibited after heat inactivation or pretreatment with RNAse, indicating a major involvement of protein and mRNA components of ES-MV in the observed phenomena. We postulate that ES-MV may efficiently expand HPC by stimulating them with ES-MV expressed ligands (e.g., Wnt-3) as well as increase their pluripotency after horizontal transfer of ES-derived mRNA.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Levine SJ . Mechanisms of soluble cytokine receptor generation. J Immunol 2004; 173: 5343–5348.
Lucas WJ, Yoo BC, Kragler F . RNA as a long-distance information macromolecule in plants. Nat Rev Mol Cell Biol 2001; 2: 849–857.
Albi E, Viola Magni MP . The role of intranuclear lipids. Biol Cell 2004; 96: 657–667.
Taback B, Hoon DS . Circulating nucleic acids and proteomics of plasma/serum: clinical utility. Ann N Y Acad Sci 2004; 1022: 1–8.
Janowska-Wieczorek A, Majka M, Kijowski J, Baj-Krzyworzeka M, Reca R, Turner AR et al. Platelet-derived microparticles bind to hematopoietic progenitor cells and enhance their engraftment. Blood 2001; 98: 3143–3149.
Baj-Krzyworzeka M, Majka M, Pratico D, Ratajczak J, Vilaire G, Kijowski J et al. Platelet-derived microparticles stimulate proliferation, survival, adhesion, and chemotaxis of hematopoietic cells. Exp Hematol 2002; 30: 450–459.
Morel O, Toti F, Hugel B, Freyssinet JM . Cellular microparticles: a disseminated storage pool of bioactive vascular effectors. Curr Opin Hematol 2004; 11: 156–164.
Christian JL . Argosomes: intracellular transport vehicles for intercellular signals? Sci STKE 2002; 2002: PE13.
Heijnen HF, Schiel AE, Fijnheer R, Geuze HJ, Sixma JJ . Activated platelets release two types of membrane vesicles: microvesicles by surface shedding and exosomes derived from exocytosis of multivesicular bodies and alpha-granules. Blood 1999; 94: 3791–3799.
Nomura S, Nakamura T, Cone J, Tandon NN, Kambayashi J . Cytometric analysis of high shear-induced platelet microparticles and effect of cytokines on microparticle generation. Cytometry 2000; 40: 173–181.
VanWijk MJ, VanBavel E, Sturk A, Nieuwland R . Microparticles in cardiovascular diseases. Cardiovasc Res 2003; 59: 277–287.
Rozmyslowicz T, Majka M, Kijowski J, Murphy SL, Conover DO, Poncz M et al. Platelet- and megakaryocyte-derived microparticles transfer CXCR4 receptor to CXCR4-null cells and make them susceptible to infection by X4-HIV. AIDS 2003; 17: 33–42.
Janowska-Wieczorek A, Wysoczynski M, Kijowski J, Marquez-Curtis L, Machalinski B, Ratajczak J et al. Microvesicles derived from activated platelets induce metastasis and angiogenesis in lung cancer. Int J Cancer 2005; 113: 752–760.
Fackler OT, Peterlin BM . Endocytic entry of HIV-1. Curr Biol 2000; 10: 1005–1008.
Fevrier B, Vilette D, Archer F, Loew D, Faigle W, Vidal M et al. Cells release prions in association with exosomes. Proc Natl Acad Sci USA 2004; 101: 9683–9688.
Gould SJ, Booth AM, Hildreth JE . The Trojan exosome hypothesis. Proc Natl Acad Sci USA 2003; 100: 10592–10597.
Cowan CA, Atienza J, Melton DA, Eggan K . Nuclear reprogramming of somatic cells after fusion with human embryonic stem cells. Science 2005; 309: 1369–1373.
Do JT, Scholer HR . Nuclei of embryonic stem cells reprogram somatic cells. Stem Cells 2004; 22: 941–949.
Landsverk HB, Hakelien AM, Kuntziger T, Robl JM, Skalhegg BS, Collas P . Reprogrammed gene expression in a somatic cell-free extract. EMBO Rep 2002; 3: 384–389.
Western PS, Surani MA . Nuclear reprogramming – alchemy or analysis? Nat Biotechnol 2002; 20: 445–446.
Wysoczynski M, Reca R, Ratajczak J, Kucia M, Shirvaikar N, Honczarenko M et al. Incorporation of CXCR4 into membrane lipid rafts primes homing-related responses of hematopoietic progenitor cells to an SDF-1 gradient. Blood 2005; 105: 40–48.
Ratajczak J, Reca R, Kucia M, Majka M, Allendorf DJ, Baran JT et al. Mobilization studies in mice deficient in either C3 or C3a receptor (C3aR) reveal a novel role for complement in retention of hematopoietic progenitor cells in bone marrow. Blood 2004; 103: 2071–2078.
Majka M, Janowska-Wieczorek A, Ratajczak J, Ehrenman K, Pietrzkowski Z, Kowalska MA et al. Numerous growth factors, cytokines, and chemokines are secreted by human CD34(+) cells, myeloblasts, erythroblasts, and megakaryoblasts and regulate normal hematopoiesis in an autocrine/paracrine manner. Blood 2001; 97: 3075–3085.
Willert K, Brown JD, Danenberg E, Duncan AW, Weissman IL, Reya T et al. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature 2003; 423: 448–452.
Reya T, Duncan AW, Ailles L, Domen J, Scherer DC, Willert K et al. A role for Wnt signalling in self-renewal of haematopoietic stem cells. Nature 2003; 423: 409–414.
Sato N, Meijer L, Skaltsounis L, Greengard P, Brivanlou AH . Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nat Med 2004; 10: 55–63.
Halicka HD, Bedner E, Darzynkiewicz Z . Segregation of RNA and separate packaging of DNA and RNA in apoptotic bodies during apoptosis. Exp Cell Res 2000; 260: 248–256.
Ceccarini M, Guidoni L, Luciani AM, Mariutti G, Rosi A, Viti V . Biochemical and NMR studies on structure and release conditions of RNA-containing vesicles shed by human colon adenocarcinoma cells. Int J Cancer 1989; 44: 714–721.
Bergsmedh A, Szeles A, Henriksson M, Bratt A, Folkman MJ, Spetz AL et al. Horizontal transfer of oncogenes by uptake of apoptotic bodies. Proc Natl Acad Sci USA 2001; 98: 6407–6411.
Bergsmedh A, Szeles A, Spetz AL, Holmgren L . Loss of the p21(Cip1/Waf1) cyclin kinase inhibitor results in propagation of horizontally transferred DNA. Cancer Res 2002; 62: 575–579.
Holmgren L, Szeles A, Rajnavolgyi E, Folkman J, Klein G, Ernberg I et al. Horizontal transfer of DNA by the uptake of apoptotic bodies. Blood 1999; 93: 3956–3963.
de la Taille A, Chen MW, Burchardt M, Chopin DK, Buttyan R . Apoptotic conversion: evidence for exchange of genetic information between prostate cancer cells mediated by apoptosis. Cancer Res 1999; 59: 5461–5463.
Hakelien AM, Landsverk HB, Robl JM, Skalhegg BS, Collas P . Reprogramming fibroblasts to express T-cell functions using cell extracts. Nat Biotechnol 2002; 20: 460–466.
Graves LE, Ariztia EV, Navari JR, Matzel HJ, Stack MS, Fishman DA . Proinvasive properties of ovarian cancer ascites-derived membrane vesicles. Cancer Res 2004; 64: 7045–7049.
Greco V, Hannus M, Eaton S . Argosomes: a potential vehicle for the spread of morphogens through epithelia. Cell 2001; 106: 633–645.
Speck RF, Esser U, Penn ML, Eckstein DA, Pulliam L, Chan SY et al. A trans-receptor mechanism for infection of CD4-negative cells by human immunodeficiency virus type 1. Curr Biol 1999; 9: 547–550.
Cohen RS . The role of membranes and membrane trafficking in RNA localization. Biol Cell 2005; 97: 5–18.
Larrabee PB, Johnson KL, Lai C, Ordovas J, Cowan JM, Tantravahi U et al. Global gene expression analysis of the living human fetus using cell-free messenger RNA in amniotic fluid. JAMA 2005; 293: 836–842.
Rustom A, Saffrich R, Markovic I, Walther P, Gerdes HH . Nanotubular highways for intercellular organelle transport. Science 2004; 303: 1007–1010.
Vidulescu C, Clejan S, O'Connor KC . Vesicle traffic through intercellular bridges in DU 145 human prostate cancer cells. J Cell Mol Med 2004; 8: 388–396.
Distler JH, Jungel A, Huber LC, Seemayer CA, Reich III CF, Gay RE et al. The induction of matrix metalloproteinase and cytokine expression in synovial fibroblasts stimulated with immune cell microparticles. Proc Natl Acad Sci USA 2005; 102: 2892–2897.
Jang YY, Collector MI, Baylin SB, Diehl AM, Sharkis SJ . Hematopoietic stem cells convert into liver cells within days without fusion. Nat Cell Biol 2004; 6: 532–539.
Acknowledgements
This work was supported by NIH Grant R01 CA106281-01 to MZR and a GA CR 301/03/1122 Grant to PD.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ratajczak, J., Miekus, K., Kucia, M. et al. Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: evidence for horizontal transfer of mRNA and protein delivery. Leukemia 20, 847–856 (2006). https://doi.org/10.1038/sj.leu.2404132
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.leu.2404132
Keywords
This article is cited by
-
Non-bone-derived exosomes: a new perspective on regulators of bone homeostasis
Cell Communication and Signaling (2024)
-
Extracellular lipidosomes containing lipid droplets and mitochondria are released during melanoma cell division
Cell Communication and Signaling (2024)
-
Extracellular vesicles, genetic programmers
Nature Cell Biology (2024)
-
Leukemogenesis occurs in a microenvironment enriched by extracellular microvesicles/exosomes: recent discoveries and questions to be answered
Leukemia (2024)
-
Engineering of Cell Derived-Nanovesicle as an Alternative to Exosome Therapy
Tissue Engineering and Regenerative Medicine (2024)