Abstract
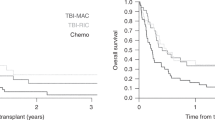
To evaluate the results of autologous stem cell transplantation (ASCT) in a large population of adults with acute lymphoblastic leukemia (ALL) in first complete remission (CR), we performed an individual data-based overview of the last three trials from the LALA group. Overall, 349 patients with ALL prospectively randomized in the consecutive LALA-85, -87, and -94 trials to receive either ASCT or chemotherapy as post-CR treatment were analyzed. Eligibility criteria were 15–50-year-old patients without sibling donors in both LALA-85/87 trials and 15–55-year-old patients with high-risk ALL and no sibling donors in the LALA-94 trial. Intent-to-treat analysis, which compared 175 patients from the ASCT arm to 174 patients from the chemotherapy arm, showed that ASCT was associated with a lower cumulative incidence of relapse (66 vs 78% at 10 years; P=0.05), without significant gain in disease-free or overall survival. Despite a possible lack of statistical power, a nested case–control analysis performed in 85 patient pairs adjusted for time to transplant and prognostic covariates confirmed these intent-to-treat results in patients actually transplanted. Of interest, the reduced relapse risk after ASCT translated in better disease-free survival in the 300 rapid responders who reached CR after the first induction course.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Fiere D, Archimbaud E, Extra JM, Marty M, David B, Witz F et al. Treatment of adult acute lymphoblastic leukemia. Preliminary results of a trial from the French Group. Haematol Blood Transfus 1987; 30: 125–129.
Fiere D, Lepage E, Sebban C, Boucheix C, Gisselbrecht C, Vernant JP et al. Adult acute lymphoblastic leukemia: a multicentric randomized trial testing bone marrow transplantation as postremission therapy. The French Group on Therapy for Adult Acute Lymphoblastic Leukemia. J Clin Oncol 1993; 11: 1990–2001.
Nagura E, Kimura K, Yamada K, Ota K, Maekawa T, Takaku F et al. Nation-wide randomized comparative study of doxorubicin, vincristine and prednisolone combination therapy with and without L-asparaginase for adult acute lymphoblastic leukemia. Cancer Chemother Pharmacol 1994; 33: 359–365.
Larson RA, Dodge RK, Linker CA, Stone RM, Powell BL, Lee EJ et al. A five-drug remission induction regimen with intensive consolidation for adults with acute lymphoblastic leukemia: Cancer and Leukemia Group B study 8811. Blood 1995; 85: 2025–2037.
Dekker AW, van't Veer MB, Sizoo W, Haak HL, van der Lelie J, Ossenkoppele G et al. Intensive postremission chemotherapy without maintenance therapy in adults with acute lymphoblastic leukemia. Dutch Hemato-Oncology Research Group. J Clin Oncol 1997; 15: 476–482.
Durrant IJ, Prentice HG, Richards SM . Intensification of treatment for adults with acute lymphoblastic leukaemia: results of UK. Medical Research Council randomized trial UKALL XA. Medical Research Council Working Party on Leukaemia in Adults. Br J Haematol 1997; 99: 84–92.
Ribera JM, Ortega JJ, Oriol A, Fontanillas M, Hernandez-Rivas JM, Brunet S et al. Late intensification chemotherapy has not improved the results of intensive chemotherapy in adult acute lymphoblastic leukemia. Results of a prospective multicenter randomized trial (PETHEMA ALL-89). Haematologica 1998; 83: 222–230.
Larson RA, Dodge RK, Linker CA, Stone RM, Powell BL, Lee EJ et al. A randomized controlled trial of filgrastim during remission induction and consolidation chemotherapy for adults with acute lymphoblastic leukemia: CALGB study 9111. Blood 1998; 92: 1556–1564.
Kantarjian HM, O'Brien S, Smith TL, Cortes J, Giles FJ, Beran M et al. Results of treatment with hyper-CVAD, a dose-intensive regimen, in adult acute lymphocytic leukemia. J Clin Oncol 2000; 18: 547–561.
Gokbuget N, Hoelzer D, Arnold R, Bohme A, Bartram CR, Freund M et al. Treatment of Adult ALL according to protocols of the German Multicenter Study Group for Adult ALL (GMALL). Hematol Oncol Clin North Am 2000; 14: 1307–1325.
Thiebaut A, Vernant JP, Degos L, Huguet FR, Reiffers J, Sebban C et al. Adult acute lymphocytic leukemia study testing chemotherapy and autologous and allogeneic transplantation. A follow-up report of the French protocol LALA 87. Hematol Oncol Clin North Am 2000; 14: 1353–1366.
Annino L, Vegna ML, Camera A, Specchia G, Visani G, Fioritoni G et al. Treatment of adult acute lymphoblastic leukemia (ALL): long-term follow- up of the GIMEMA ALL 0288 randomized study. Blood 2002; 99: 863–871.
Rowe JM, Buck G, Burnett AK, Chopra R, Wiernik PH, Richards SM et al. Induction therapy for adults with acute lymphoblastic leukemia: results of more than 1,500 patients from the international ALL Trial: MRC UKALL XII/ECOG E2993. Blood 2005; 106: 3760–3767.
Attal M, Blaise D, Marit G, Payen C, Michallet M, Vernant JP et al. Consolidation treatment of adult acute lymphoblastic leukemia: a prospective, randomized trial comparing allogeneic versus autologous bone marrow transplantation and testing the impact of recombinant interleukin-2 after autologous bone marrow transplantation. BGMT Group. Blood 1995; 86: 1619–1628.
Sebban C, Lepage E, Vernant JP, Gluckman E, Attal M, Reiffers J et al. Allogeneic bone marrow transplantation in adult acute lymphoblastic leukemia in first complete remission: a comparative study. French Group of Therapy of Adult Acute Lymphoblastic Leukemia. J Clin Oncol 1994; 12: 2580–2587.
Hunault M, Harousseau JL, Delain M, Truchan-Graczyk M, Cahn JY, Witz F et al. Better outcome of adult acute lymphoblastic leukemia after early genoidentical allogeneic bone-marrow transplantation (BMT) than after late high-dose therapy and autologous BMT: a GOELAMS trial. Blood 2004; 104: 3028–3037.
Martin TG, Linker CA . Autologous stem cell transplantation for acute lymphocytic leukemia in adults. Hematol Oncol Clin North Am 2001; 15: 121–143.
Weisdorf DJ, Billett AL, Hannan P, Ritz J, Sallan SE, Steinbuch M et al. Autologous versus unrelated donor allogeneic marrow transplantation for acute lymphoblastic leukemia. Blood 1997; 90: 2962–2968.
Vey N, Blaise D, Stoppa AM, Bouabdallah R, Lafage M, Sainty D et al. Bone marrow transplantation in 63 adult patients with acute lymphoblastic leukemia in first complete remission. Bone Marrow Transplant 1994; 14: 383–388.
Granena A, Castellsague X, Badell I, Ferra C, Ortega J, Brunet S et al. Autologous bone marrow transplantation for high risk acute lymphoblastic leukemia: clinical relevance of ex vivo bone marrow purging with monoclonal antibodies and complement. Bone Marrow Transplant 1994; 24: 621–627.
Gorin NC, Aegerter P, Auvert B . Autologous bone marrow transplantation for acute leukemia in remission: an analysis of 1322 cases. Haematol Blood Transfus 1990; 33: 660–666.
Powles R, Mehta J, Singhal S, Horton C, Tait D, Milan S et al. Autologous bone marrow or peripheral blood stem cell transplantation followed by maintenance chemotherapy for adult acute lymphoblastic leukemia in first remission: 50 cases from a single center. Bone Marrow Transplant 1995; 16: 241–247.
Cahn JY, Bordigoni P, Souillet G, Pico JL, Plouvier E, Reiffers J et al. The TAM regimen prior to allogeneic and autologous bone marrow transplantation for high-risk acute lymphoblastic leukemias: a cooperative study of 62 patients. Bone Marrow Transplant 1991; 7: 1–4.
Blaise D, Attal M, Pico JL, Reiffers J, Stoppa AM, Bellanger C et al. The use of a sequential high dose recombinant interleukin 2 regimen after autologous bone marrow transplantation does not improve the disease free survival of patients with acute leukemia transplanted in first complete remission. Leuk Lymphoma 1997; 25: 469–478.
Gorin NC, Labopin M, Laporte JP, Douay L, Lopez M, Lesage S et al. Importance of marrow dose on posttransplant outcome in acute leukemia: models derived from patients autografted with mafosfamide-purged marrow at a single institution. Exp Hematol 1999; 27: 1822–1830.
Thomas X, Boiron JM, Huguet F, Dombret H, Bradstock K, Vey N et al. Outcome of treatment in adults with acute lymphoblastic leukemia: analysis of the LALA-94 trial. J Clin Oncol 2004; 22: 4075–4086.
Ribera JM, Oriol A, Bethencourt C, Parody R, Hernandez-Rivas JM, Moreno MJ et al. Comparison of intensive chemotherapy, allogeneic or autologous stem cell transplantation as post-remission treatment for adult patients with high-risk acute lymphoblastic leukemia. Results of the PETHEMA ALL-93 trial. Haematologica 2005; 90: 1346–1356.
Labar B, Suciu S, Zittoun R, Muus P, Marie JP, Fillet G, et al., EORTC Leukemia Group. Allogeneic stem cell transplantation in acute lymphoblastic leukemia and non-Hodgkin's lymphoma for patients ⩽50 years old in first complete remission: results of the EORTC ALL-3 trial. Haematologica 2004; 89: 809–817.
Fiere D, Broustet A, Leblond V, Maraninchi D, Castaigne S, Flesch M et al. Comparison of chemotherapy and autologous and allogeneic transplantation as postinduction regimen in adult acute lymphoblastic leukemia: a preliminary multicentric study. Hematol Blood Transf 1990; 33: 409–412.
Bennett JM, Catovsky D, Daniel MT, Flandrin G, Galton DA, Gralnick HR et al. Proposals for the classification of the acute leukaemias. French–American–British (FAB) cooperative group. Br J Haematol 1976; 33: 451–458.
ISCN (International System for Human Cytogenetic Nomenclature). Guidelines for Cancer Cytogenetics. In: Mittelman F (ed). Supplement to an International System for Human Cytogenetic Nomenclature. Karger: Basel, 1991, pp 1–53.
Gaynor J, Chapman D, Little C, McKenzie S, Miller W, Andreeff M et al. A cause-specific hazard rate analysis of prognostic factors among 199 adults with acute lymphoblastic leukemia: the Memorial Hospital experience since 1969. J Clin Oncol 1988; 6: 1014–1030.
Gooley T, Leisenring W, Crowley J, Storer B . Why Kaplan-Meier fails and cumulative incidence succeeds when estimating failures probabilities in the presence of competing risks. In: Crowley J (ed). Handbook of Statistics in Clinical Oncology. Marcel Dekker: New York, 2001, pp 513–523.
Hess KR . Graphical methods for assessing violations of the proportional hazards assumption in Cox regression. Stat Med 1995; 14: 1707–1723.
Cox D . Regression models and life tables. J R Stat Soc 1972; 34: 187–220.
Mortuza FY, Papaionnaou M, Moreira IL, Coyle LA, Gameiro P, Gandini D et al. Minimal residual disease test provide a independent predictor of clinical outcome in adult acute lymphoblastic leukemia. J Clin Oncol 2002; 20: 1094–1104.
Dombret H, Gabert J, Boiron JM, Rigal-Huguet F, Blaise D, Thomas X et al. Outcome of treatment in adults with Philadelphia chromosome-positive acute lymphoblastic leukemia – results of the prospective multicenter LALA-94 trial. Blood 2002; 100: 2357–2366.
Meloni G, Diverio D, Vignetti M, Avvisati G, Capria S, Petti MC et al. Autologous bone marrow transplantation for acute promyelocytic leukemia in second remission: prognostic relevance of pretransplant minimal residual disease assessment by reverse-transcription polymerase chain reaction of the PML/RARα fusion gene. Blood 1997; 90: 1321–1325.
Powles R, Sirohi B, Treleaven J, Kulkarni S, Tait D, Singhal S et al. The role of post-transplantation maintenance chemotherapy in improving the outcome of auto-transplantation in adult acute lymphoblastic leukemia. Blood 2002; 100: 1641–1647.
Ottmann OG, Druker BJ, Sawyers CL, Goldman JM, Reiffers J, Silver RT et al. A phase 2 study of imatinib in patients with relapsed or refractory Philadelphia chromosome-positive acute lymphoid leukemias. Blood 2002; 100: 1965–1971.
Thomas DA, Faderl S, Cortes J, O'Brien S, Giles FJ, Kornblau SM et al. Treatment of Philadelphia chromosome-positive acute lymphocytic leukemia with hyper-CVAD and imatinib mesylate. Blood 2004; 103: 4396–4407.
Towatari M, Yanada M, Usui N, Takeuchi J, Sugiura I, Takeuchi M, et al., (Japan Adult Leukemia Study Group). Combination of intensive chemotherapy and imatinib can rapidly induce high-quality complete remission for a majority of patients with newly diagnosed BCR-ABL positive acute lymphoblastic leukemia. Blood 2004; 104: 3507–3512.
Lee KH, Lee JH, Choi SJ, Lee JH, Seol M, Lee YS et al. Clinical effect of imatinib added to intensive combination chemotherapy for newly diagnosed Philadelphia chromosome-positive acute lymphoblastic leukemia. Leukemia 2005; 19: 1509–1516.
Acknowledgements
We are indebted to Wim van Putten for providing Stata packages with facilities for Kaplan–Meier survival and cumulative incidence curves. This study was supported in part by Le Programme Hospitalier de Recherche Clinique (PHRC N°94-95-97.02), Ministère de l'Emploi et de la Solidarité, France; and by L'Association Contre le Cancer (National Grants ARC Nos. 6237, 9623, and 5484).
Author information
Authors and Affiliations
Consortia
Corresponding author
Appendix
Appendix
French participating centers were: Hôpital Pitié-Salpétrière, Paris; Hôpital Saint-Louis, Paris; Hôpital Edouard Herriot, Lyon; Hôpital du Haut Lévêque, Bordeaux; Hôpital Purpan, Toulouse; Hôpital André Mignot, Versailles; Hôpital Henri Mondor, Créteil; Hôpital Bicètre, Le Kremlin Bicètre; Institut Paoli Calmettes, Marseille; Hôpital Cochin, Paris; Hôpital de Hautepierre, Strasbourg; EFS, Besançon; Hôpital de l'Archet, Nice; Hôpital Necker, Paris; Hôpital Jean Bernard, Poitiers; Hôpital Michallon, Grenoble; Institut Gustave Roussy, Villejuif; INSERM U268, Villejuif; Hôpital du Bocage, Dijon; Hôpital Saint-Antoine, Paris; Centre Hospitalier, Caen; Hôpital Pontchaillou, Rennes; Centre Hospitalier de la Côte Basque, Bayonne; Centre Hospitalier, Lyon; HIA Percy, Clamart; HIA du Val-de-Grâce, Paris; Centre Hospitalier, Chambéry; Hôpital Dupuytren, Limoges; Centre Hospitalier, Avignon; Hôpital Louis Pasteur, Colmar; Centre Hospitalier, Mulhouse; Centre Henri Becquerel, Rouen; Centre Hospitalier, Clermont-Ferrand; Centre Hospitalier, Lille; Hôpital Beaujon, Clichy; Centre Hospitalier, Annecy; Centre Hospitalier, Perpignan; Centre Hospitalier Lapeyronie, Montpellier; Centre Hospitalier, Aix en Provence; Hôpital Jean Monod, Le Havre; Centre Hospitalier, Meaux; Hôpital Hôtel Dieu, Paris; Laboratoire CERBA, Val d'Oise; Hôpital Lariboisière, Paris; Hôpital Victor Dupouy, Argenteuil; Centre Hospitalier Dr Schaffner, Lens; Centre Hospitalier, Valenciennes; Centre Hospitalier Saint-Vincent, Lille; Centre Hospitalier, Roubaix; and ETS, Lille. Belgium participating centers were: Cliniques St Luc, Bruxelles; Centre Hospitalier Notre Dame et Reine Fabiola, Charleroi; Cliniques de Mont Godinne, Yvoir; ASBL, Loverval; Hôpital Saint-Joseph, Gilly; Hôpital de Jolimont, Haine St Paul; Hôpital Saint-Joseph, Mons; Hôpital de la Citadelle, Liège; and Laboratoire de Cytogénétique, Leuven. Swiss participating centers were: Centre Hospitalier Universitaire Vaudois, Lausanne; Kantonsspital, St Gallen; Universitätsspital, Zürich; Hôpital Cantonal Universitaire, Genève; Kantonsspital, Basel; Inselspital, Bern; Kantonsspital, Winterthur; Kantonsspital, Luzern, Switzerland. Australian participating centers were: Westmead Hospital, Westmead; Adelaide Hospital, Adelaide; Mater Misericordae Hospital, Newcastle; Alfred Hospital, Melbourne; Royal Adelaide Hospital, Adelaide; Peter Mac Callum Cancer Centre Institute, Melbourne; Monash Medical Centre, Melbourne; Liverpool Hospital, Sydney; St George Hospital, Sydney, Australia.
Rights and permissions
About this article
Cite this article
Dhédin, N., Dombret, H., Thomas, X. et al. Autologous stem cell transplantation in adults with acute lymphoblastic leukemia in first complete remission: analysis of the LALA-85, -87 and -94 trials. Leukemia 20, 336–344 (2006). https://doi.org/10.1038/sj.leu.2404065
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.leu.2404065
Keywords
This article is cited by
-
Propensity score matching/reweighting analysis comparing autologous and allogeneic stem cell transplantation for B-lineage acute lymphoblastic leukemia
International Journal of Hematology (2022)
-
An auto-SCT-based total therapy resulted in encouraging outcomes in adolescents and young adults with acute lymphoblastic leukemia: report from a single center of China
Bone Marrow Transplantation (2012)
-
Autologous hematopoietic cell transplantation for high-risk ALL
Bone Marrow Transplantation (2011)
-
Status of minimal residual disease determines outcome of autologous hematopoietic SCT in adult ALL
Bone Marrow Transplantation (2010)
-
Autologous hematopoietic stem cell transplantation in adult acute lymphoblastic leukemia: still not out of fashion
Annals of Hematology (2009)