Abstract
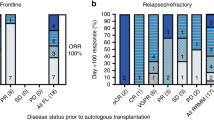
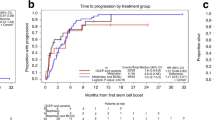
Autologous stem cell transplantation (SCT) with high-dose melphalan (HDM, 200 mg/m2) is the most effective therapy for multiple myeloma. To determine the feasibility of combining carmustine (300 mg/m2) with HDM, we enrolled 49 patients with previously treated Durie–Salmon stage II/III myeloma (32M/17W, median age 53) on a phase I/II trial involving escalating doses of melphalan (160, 180, 200 mg/m2). The median β2-microglobulin was 2.5 (0–9.3); marrow karyotypes were normal in 88%. The phase I dose-limiting toxicity was ⩾grade 2 pulmonary toxicity 2 months post-SCT. Other endpoints were response rate and progression-free survival (PFS). HDM was safely escalated to 200 mg/m2; treatment-related mortality was 2% and ⩾grade 2 pulmonary toxicity 10%. The complete (CR) and near complete (nCR) response rate was 49%. With a median post-SCT follow-up of 2.9 years, the PFS and overall survival (OS) post-SCT were 2.3 and 4.7 years. PFS for those with CR or nCR was 3.1 years while for those with stable disease (SD) it was 1.3 years (P=0.06). We conclude that carmustine can be combined with HDM for myeloma with minimal pulmonary toxicity and a high response rate.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Barlogie B, Shaughnessy J, Tricot G, Jacobson J, Zangari M, Anaissie E et al. Treatment of multiple myeloma. Blood 2004; 103: 20–32.
Child JA, Morgan GJ, Davies FE, Owen RG, Bell SE, Hawkins K et al. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med 2003; 348: 1875–1883.
Attal M, Harousseau JL, Stoppa AM, Sotto JJ, Fuzibet JG, Rossi JF et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Francais du Myelome. N Engl J Med 1996; 335: 91–97.
Gulbrandsen N, Wisloff F, Brinch L, Carlson K, Dahl IM, Gimsing P et al. Health-related quality of life in multiple myeloma patients receiving high-dose chemotherapy with autologous blood stem-cell support. Med Oncol 2001; 18: 65–77.
Lenhoff S, Hjorth M, Holmberg E, Turesson I, Westin J, Nielsen JL et al. Impact on survival of high-dose therapy with autologous stem cell support in patients younger than 60 years with newly diagnosed multiple myeloma: a population-based study. Nordic Myeloma Study Group. Blood 2000; 95: 7–11.
Hahn T, Wingard JR, Anderson KC, Bensinger WI, Berenson JR, Brozeit G et al. The role of cytotoxic therapy with hematopoietic stem cell transplantation in the therapy of multiple myeloma. An evidence-based review. Biol Blood Marrow Transplant 2003; 9: 4–37.
Desikan R, Barlogie B, Sawyer J, Ayers D, Tricot G, Badros A et al. Results of high-dose therapy for 1000 patients with multiple myeloma: durable complete remissions and superior survival in the absence of chromosome 13 abnormalities. Blood 2000; 95: 4008–4010.
Tricot G, Spencer T, Sawyer J, Spoon D, Desikan R, Fassas A et al. Predicting long-term (> or =5 years) event-free survival in multiple myeloma patients following planned tandem autotransplants. Br J Haematol 2002; 116: 211–217.
Fassas AB, Spencer T, Sawyer J, Zangari M, Lee CK, Anaissie E et al. Both hypodiploidy and deletion of chromosome 13 independently confer poor prognosis in multiple myeloma. Br J Haematol 2002; 118: 1041–1047.
Soverini S, Cavo M, Cellini C, Terragna C, Zamagni E, Ruggeri D et al. Cyclin D1 overexpression is a favorable prognostic variable for newly diagnosed multiple myeloma patients treated with high-dose chemotherapy and single or double autologous transplantation. Blood 2003; 102: 1588–1594.
Desikan KR, Tricot G, Dhodapkar M, Fassas A, Siegel D, Vesole DH et al. Melphalan plus total body irradiation (MEL-TBI) or cyclophosphamide (MEL-CY) as a conditioning regimen with second autotransplant in responding patients with myeloma is inferior compared to historical controls receiving tandem transplants with melphalan alone. Bone Marrow Transplant 2000; 25: 483–487.
Moreau P, Facon T, Attal M, Hulin C, Michallet M, Maloisel F et al. Comparison of 200 mg/m2 melphalan and 8 Gy total body irradiation plus 140 mg/m2 melphalan as conditioning regimens for peripheral blood stem cell transplantation in patients with newly diagnosed multiple myeloma: nal analysis of the Intergroupe Francophone du Myelome 9505 randomized trial. Blood 2002; 99: 731–735.
Lahuerta JJ, Grande C, Blade J, Martinez-Lopez J, de la Serna J, Alegre A et al. Myeloablative treatments for multiple myeloma: update of a comparative study of different regimens used in patients from the Spanish registry for transplantation in multiple myeloma. Leuk Lymphoma 2002; 43: 67–74.
Ager S, Mahendra P, Richards EM, Bass G, Baglin TP, Marcus RE . High-dose carmustine, etoposide and melphalan (‘BEM’) with autologous stem cell transplantation: a dose-toxicity study. Bone Marrow Transplant 1996; 17: 335–340.
Bailey CC, Marsden HB, Jones PH . Fatal pulmonary fibrosis following 1,3-bis(2-chloroethyl)-1-nitrosourea (BCNU) therapy. Cancer 1978; 42: 74–76.
Abushamaa AM, Sporn TA, Folz RJ . Oxidative stress and inflammation contribute to lung toxicity after a common breast cancer chemotherapy regimen. Am J Physiol Lung Cell Mol Physiol 2002; 283: L336–L345.
Wheeler C, Antin JH, Churchill WH, Come SE, Smith BR, Bubley GJ et al. Cyclophosphamide, carmustine, and etoposide with autologous bone marrow transplantation in refractory Hodgkin's disease and non-Hodgkin's lymphoma: a dose-nding study. J Clin Oncol 1990; 8: 648–656.
Kersiene R, Maroziene A, Kliukiene R, Anusevicius Z, Didziapetriene J, Cenas N . Modulation of erythrocyte photohemolysis rate by glutathione reductase inactivating alkylating agents. Biochem Mol Biol Int 1998; 45: 709–716.
Samuels BL, Bitran JD . High-dose intravenous melphalan: a review. J Clin Oncol 1995; 13: 1786–1799.
Hamlin PA, Zelenetz AD, Kewalramani T, Qin J, Satagopan JM, Verbel D et al. Age-adjusted International Prognostic Index predicts autologous stem cell transplantation outcome for patients with relapsed or primary refractory diffuse large B-cell lymphoma. Blood 2003; 102: 1989–1996.
Moskowitz CH, Kewalramani T, Nimer SD, Gonzalez M, Zelenetz AD, Yahalom J . Effectiveness of high dose chemoradiotherapy and autologous stem cell transplantation for patients with biopsy-proven primary refractory Hodgkin's disease. Br J Haematol 2004; 124: 645–652.
Gupta S, Zhou P, Hassoun H, Kewalramani T, Reich L, Costello S et al. Hematopoietic stem cell mobilization with intravenous melphalan and G-CSF in patients with chemoresponsive multiple myeloma: report of a phase II trial. Bone Marrow Transplant 2005; 35: 441–447.
Trotti A, Byhardt R, Stetz J, Gwede C, Corn B, Fu K et al. Common toxicity criteria: version 2.0 an improved reference for grading the acute effects of cancer treatment: impact on radiotherapy. Int J Radiat Oncol Biol Phys 2000; 47: 13–47.
Woodruff RK, Wadsworth J, Malpas JS, Tobias JS . Clinical staging in multiple myeloma. Br J Haematol 1979; 42: 199–205.
Greipp PR, San Miguel J, Durie BG, Crowley JJ, Barlogie B, Blade J et al. International staging system for multiple myeloma. J Clin Oncol 2005; 23: 3412–3420.
Blade J, Samson D, Reece D, Apperley J, Bjorkstrand B, Gahrton G et al. Criteria for evaluating disease response and progression in patients with multiple myeloma treated by high-dose therapy and haemopoietic stem cell transplantation. Myeloma Subcommittee of the EBMT. European Group for Blood and Marrow Transplant. Br J Haematol 1998; 102: 1115–1123.
Abraham R, Chen C, Tsang R, Simpson D, Murray C, Davidson M et al. Intensification of the stem cell transplant induction regimen results in increased treatment-related mortality without improved outcome in multiple myeloma. Bone Marrow Transplant 1999; 24: 1291–1297.
Porrata LF, Gertz MA, Geyer SM, Litzow MR, Gastineau DA, Moore SB et al. The dose of infused lymphocytes in the autograft directly correlates with clinical outcome after autologous peripheral blood hematopoietic stem cell transplantation in multiple myeloma. Leukemia 2004; 18: 1085–1092.
Jagannath S, Richardson PG, Sonneveld P, Irwin D, Schuster MW, Stadtmauer EA et al. Bortezomib appears to overcome poor prognosis conferred by chromosome 13 deletion in phase 2 and 3 trials. J Clin Oncol 2005; 23: 6501a.
Shimoni A, Smith TL, Aleman A, Weber D, Dimopoulos M, Anderlini P et al. Thiotepa, busulfan, cyclophosphamide (TBC) and autologous hematopoietic transplantation: an intensive regimen for the treatment of multiple myeloma. Bone Marrow Transplant 2001; 27: 821–828.
Moreau P, Milpied N, Mahe B, Juge-Morineau N, Rapp MJ, Bataille R et al. Melphalan 220 mg/m2 followed by peripheral blood stem cell transplantation in 27 patients with advanced multiple myeloma. Bone Marrow Transplant 1999; 23: 1003–1006.
Phillips GL, Meisenberg B, Reece DE, Adams VR, Badros A, Brunner J et al. Amifostine and autologous hematopoietic stem cell support of escalating-dose melphalan: A phase I study. Biol Blood Marrow Transplant 2004; 10: 473–483.
Giralt S, Bensinger W, Goodman M, Podoloff D, Eary J, Wendt R et al. 166Ho-DOTMP plus melphalan followed by peripheral blood stem cell transplantation in patients with multiple myeloma: results of two phase 1/2 trials. Blood 2003; 102: 2684–2691.
Williams L, Beveridge RA, Rifkin RM, Bretzel RL, Cuasay L, Alley JL et al. Increased pulmonary toxicity results from a 1-day versus 2-day schedule of administration of high-dose melphalan. Biol Blood Marrow Transplant 2002; 8: 334–335.
Ho VT, Weller E, Lee SJ, Alyea EP, Antin JH, Soiffer RJ . Prognostic factors for early severe pulmonary complications after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2001; 7: 223–229.
Acknowledgements
We thank the nursing staff and nurse practitioners in the out-patient clinics and particularly on MSKCC Medical 11 for the care they provide to our patients, and the MSKCC hematology fellows who have participated in the care of these patients. Grants and Support: This work was supported by NIH Grant CA05826, FDA Grant R03-002174, the Graziano Fund, the Mel Stottlemyre Myeloma Research Fund, the Donald Stein Myeloma Research Fund, and the Werner and Elaine Dannheiser Fund for Research on the Biology of Aging of the Lymphoma Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Comenzo, R., Hassoun, H., Kewalramani, T. et al. Results of a phase I/II trial adding carmustine (300 mg/m2) to melphalan (200 mg/m2) in multiple myeloma patients undergoing autologous stem cell transplantation. Leukemia 20, 345–349 (2006). https://doi.org/10.1038/sj.leu.2404003
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.leu.2404003
Keywords
This article is cited by
-
High-dose bendamustine and melphalan conditioning for autologous stem cell transplantation for patients with multiple myeloma
Bone Marrow Transplantation (2019)
-
Tandem chemo-mobilization followed by high-dose melphalan and carmustine with single autologous hematopoietic cell transplantation for multiple myeloma
Bone Marrow Transplantation (2012)