Abstract
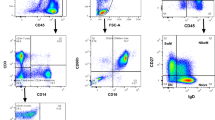
Expression of CD99 is higher on immature than on mature T cells. We postulated that this marker could be used to assess minimal residual disease (MRD) in T-lineage acute lymphoblastic leukemia (T-ALL). In diagnostic bone marrow (BM) samples from 27 children with T-ALL, expression of CD99 on leukemic lymphoblasts by flow cytometry was in median 7.7 times higher than on normal T lymphocytes from within the same sample. In 85% of cases, leukemic MFI values were higher than the mean MFI+2 s.d. of normal populations. We applied CD99 to study MRD in 39 follow-up samples from 15 consecutive T-ALL patients, and compared the results with those obtained with the well-established MRD-marker terminal deoxynucleotidyl transferase (TdT). Either antibody was combined in four-color flow cytometry with CD7, surfaceCD3, and cytoplasmicCD3. We found that CD99 was a valid complement to TdT in quantifying T-ALL MRD. Given a considerable interpatient variability, CD99 could be favorably used in nine patients, and TdT in other five patients. Both approaches showed a similar very low nonspecific background throughout 12 weeks from diagnosis (in median 0.002% of nucleated BM cells in patients with non-T ALL). We conclude that CD99 is a highly informative tool for MRD detection in T-ALL, bearing the advantage of surface expression in contrast to TdT.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Goodfellow P . Expression of the 12E7 antigen is controlled independently by genes on the human X and Y chromosomes. Differentiation 1983; 23: 35–39.
Darling SM, Goodfellow PJ, Pym B, Banting GS, Pritchard C, Goodfellow PN . Molecular genetics of MIC2: a gene shared by the human X and Y chromosomes. Cold Spring Harbor Symp Quant Biol 1986; 51: 205–212.
Dworzak MN, Fritsch G, Buchinger P, Fleischer C, Printz D, Zellner A et al. Flow cytometric assessment of human MIC2 expression in bone marrow, thymus, and peripheral blood. Blood 1994; 83: 415–425.
Kovar H, Dworzak M, Strehl S, Schnell E, Ambros IM, Ambros PF et al. Overexpression of the pseudoautosomal gene MIC2 in Ewing's sarcoma and peripheral primitive neuroectodermal tumor. Oncogene 1990; 5: 1067–1070.
Ambros IM, Ambros PF, Strehl S, Kovar H, Gadner H, Salzer-Kuntschik M . MIC2 – a specific marker for Ewing's sarcoma and peripheral primitive neuroectodermal tumors. Cancer 1991; 67: 1886–1893.
Levy R, Dilley J, Fox RI, Warnke R . A human thymus-leukemia antigen defined by hybridoma monoclonal antibodies. Proc Natl Acad Sci USA 1979; 76: 6552–6556.
Bodger MP, Francis GE, Delia D, Granger SM, Janossy G . A monoclonal antibody specific for immature human hemopoietic cells and T lineage cells. J Immunol 1981; 127: 2269–2274.
Dworzak MN, Fritsch G, Fleischer C, Printz D, Fröschl G, Buchinger P et al. CD99 (MIC2) expression in paediatric B-lineage leukaemia/lymphoma reflects maturation-associated patterns of normal B-lymphopoiesis. Br J Haematol 1999; 105: 690–695.
Schrappe M, Reiter A, Zimmermann M, Harbott J, Ludwig WD, Henze G et al. Long-term results of four consecutive trials in Berlin–Frankfurt–Münster. Leukemia 2000; 14: 2205–2222.
Van der Does-van den Berg A, Bartram CR, Basso G, Benoit YCM, Biondi A, Debatin K-M et al. Minimal requirements for the diagnosis, classification and evaluation of the treatment of childhood acute lymphoblastic leukemia (ALL) in the ‘BFM family’ cooperative group. Med Pediatr Oncol 1992; 20: 497–505.
Dworzak MN, Fröschl G, Printz D, Mann G, Pötschger U, Mühlegger N, et al, for the Austrian BFM Study Group. Prognostic significance and modalities of flow cytometric minimal residual disease detection in childhood acute lymphoblastic leukemia. Blood 2002; 99: 1952–1958.
Dworzak MN, Fritsch G, Fleischer C, Printz D, Fröschl G, Buchinger P et al. Comparative phenotype mapping of normal vs malignant pediatric B-lymphopoiesis unveils leukemia-associated aberrations. Exp Hematol 1998; 26: 305–313.
Dworzak MN, Fritsch G, Fröschl G, Printz D, Gadner H . Four-color flow cytometric investigation of terminal deoxynucleotidyl transferase-positive lymphoid precursors in pediatric bone marrow: CD79a expression precedes CD19 in early B-cell ontogeny. Blood 1998; 92: 3203–3209.
Dworzak MN, Stolz F, Fröschl G, Printz D, Henn T, Fischer S et al. Detection of residual disease in pediatric B-cell precursor acute lymphoblastic leukemia by comparative phenotype mapping: a study of five cases controlled by genetic methods. Exp Hematol 1999; 27: 673–681.
Coustan-Smith E, Behm FG, Sanchez J, Boyett JM, Hancock ML, Raimondi SC et al. Immunological detection of minimal residual disease in children with acute lymphoblastic leukaemia. Lancet 1997; 351: 550–554.
Coustan-Smith E, Sancho J, Hancock ML, Boyett JM, Behm FG, Raimondi SC et al. Clinical importance of minimal residual disease in childhood acute lymphoblastic leukemia. Blood 2000; 96: 2691–2696.
Cave H, van der Werff ten Bosch J, Suciu S, Guidal C, Waterkeyn C, Otten J, et al, for the EORTC. Clinical significance of minimal residual disease in childhood acute lymphoblastic leukemia. N Engl J Med 1998; 339: 591–598.
Van Dongen JJM, Seriu T, Panzer-Grümayer ER, Biondi A, Pongers-Willemse MJ, Corral L et al. Prognostic value of minimal residual disease in acute lymphoblastic leukaemia in childhood. Lancet 1998; 352: 1731–1738.
Bradstock KF, Papageorgiou ES, Janossy G, Hoffbrand AV, Willoughby ML, Roberts PD et al. Detection of leukaemic lymphoblasts in CSF by immunofluorescence for terminal transferase. Lancet 1980; 1: 1144.
Bradstock KF, Janossy G, Tidman N, Papageorgiou ES, Prentice HG, Willoughby M et al. Immunological monitoring of residual disease in treated thymic acute lymphoblastic leukemia. Leuk Res 1981; 5: 301–309.
Van Dongen JJM, Hooijkaas H, Adriaansen HJ, Hählen K, van Zanen GE . Detection of minimal residual acute lymphoblastic leukemia by immunological marker analysis: possibilities and limitations. In: Hagenbeek A, Löwenberg B (eds). Minimal Residual Disease in Acute Leukaemia. Dordrecht: M. Nijhoff Publishers, 1986, pp 113–133.
Hooijkaas H, Hählen K, Adriaansen HJ, Dekker I, van Zanen GE, van Dongen JJ . Terminal deoxynucleotidyl transferase (TdT)-positive cells in cerebrospinal fluid and development of overt CNS leukemia: a 5-year follow-up study in 113 children with a TdT-positive leukemia or non-Hodgkin's lymphoma. Blood 1989; 74: 416–422.
Campana D, Coustan-Smith E, Janossy G . The immunologic detection of minimal residual disease in acute leukemia. Blood 1990; 76: 163–171.
Porwit-MacDonald A, Björklund E, Lucio P, van Lochem EG, Mazur J, Parreira A et al. BIOMED-1 Concerted Action report: flow cytometric characterization of CD7+ cell subset in normal bone marrow as a basis for the diagnosis and follow-up of T cell acute lymphoblastic leukemia (T-ALL). Leukemia 2000; 14: 816–825.
Acknowledgements
This study has been promoted by the International BFM Study Group under the direction of M Schrappe. We thank the participants of the Austrian BFM-Study Group (B Ausserer, FM Fink, H Haas, N Jones, R Jones, W Kaulfersch, G Müller, I Mutz, R Ploier, K Schmitt, O Stöllinger, and C Urban) for their close collaboration in providing BM and PB samples. In this respect, we are also grateful to the oncologists and the laboratory-team of the St Anna Kinderspital (in particular to S Juhasz, R Kornmüller, E Neidhart, and U Stalze). We also thank G Fritsch, D Scharner, C Eichstill, and A Schumich from the department of flow cytometry of the CCRI for continued technical support. We thank G Mann, N Mühlegger, and J Regelsberger from the Austrian BFM Study Group documentation center for providing the clinical data and U Pötschger for help with statistical analyses.
Author information
Authors and Affiliations
Consortia
Corresponding author
Rights and permissions
About this article
Cite this article
Dworzak, M., Fröschl, G., Printz, D. et al. CD99 expression in T-lineage ALL: implications for flow cytometric detection of minimal residual disease. Leukemia 18, 703–708 (2004). https://doi.org/10.1038/sj.leu.2403303
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.leu.2403303
Keywords
This article is cited by
-
The emerging scenario of immunotherapy for T-cell Acute Lymphoblastic Leukemia: advances, challenges and future perspectives
Experimental Hematology & Oncology (2023)
-
CAR T cells targeting CD99 as an approach to eradicate T-cell acute lymphoblastic leukemia without normal blood cells toxicity
Journal of Hematology & Oncology (2021)
-
CD99 at the crossroads of physiology and pathology
Journal of Cell Communication and Signaling (2018)
-
CD99 suppresses osteosarcoma cell migration through inhibition of ROCK2 activity
Oncogene (2014)
-
Minimal residual disease in acute lymphoblastic leukemia: optimal methods and clinical relevance, pitfalls and recent approaches
Medical Oncology (2014)