Abstract
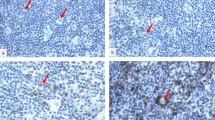
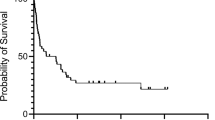
Clinical outcome in diffuse large B-cell lymphoma (DLBCL) remains unpredictable, despite the identification of clinical prognostic parameters. Here, we investigated in pretreatment biopsies of 70 patients with DLBCL whether numbers of activated cytotoxic T-lymphocytes (CTLs), as determined by the percentage of CD3-positive lymphocytes with granzyme B (GrB) expression, have similar prognostic value as found earlier in Hodgkin's lymphoma and anaplastic large-cell lymphoma and whether loss of major histocompatibility complex (MHC)-I molecules or expression of the GrB antagonist protease inhibitor 9 (PI9) may explain immune escape from CTL-mediated cell death. Independent of the International Prognostic Index (IPI), the presence of ⩾15% activated CTLs was strongly associated with failure to reach complete remission, with a poor progression-free and overall survival time. Downregulation of MHC-I light- and/or heavy-chain expression was found in 41% of interpretable cases and in 19 of 56 interpretable cases PI9 expression was detected. We conclude that a high percentage of activated CTLs is a strong, IPI independent, indicator for an unfavorable clinical outcome in patients with primary nodal DLBCL. Although in part of DLBCL expression of PI9 and loss of MHC-I expression was found, providing a possible immune-escape mechanism in these cases, no correlation with clinical outcome was found.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin's lymphoma. The Non-Hodgkin's Lymphoma Classification Project. Blood 1997; 89: 3909–3918.
Jaffe ES, Harris NL, Stein H, Vardiman JW . Pathology and Genetics of Tumors of Haematopoietic and Lymphoid Tissues. World Health Organisation Classification of Tumors. Lyon: IARC Press, 2001.
The International Non-Hodgkin's Lymphomas Prognostic Factors Project. A predictive model for aggressive non-Hodgkin's lymphoma. N Engl J Med 1993; 329: 987–994.
Oudejans JJ, Jiwa NM, Kummer JA, Ossenkoppele GJ, van Heerde P, Baars JW et al. Activated cytotoxic T cells as prognostic marker in Hodgkin's disease. Blood 1997; 89: 1376–1382.
Ten Berge RL, Dukers DF, Oudejans JJ, Pulford K, Ossenkoppele GJ, de Jong D et al. Adverse effects of activated cytotoxic T lymphocytes on the clinical outcome of nodal anaplastic large cell lymphoma. Blood 1999; 93: 2688–2696.
Johnstone RW, Ruefli AA, Lowe SW . Apoptosis: a link between cancer genetics and chemotherapy. Cell 2002; 108: 153–164.
Barry M, Bleackley RC . Cytotoxic T lymphocytes: all roads lead to death. Nat Rev Immunol 2002; 2: 401–409.
Darmon AJ, Nicholson DW, Bleackley RC . Activation of the apoptotic protease CPP32 by cytotoxic T-cell-derived granzyme B. Nature 1995; 377: 446–448.
Darmon AJ, Ley TJ, Nicholson DW, Bleackley RC . Cleavage of CPP32 by granzyme B represents a critical role for granzyme B in the induction of target cell DNA fragmentation. J Biol Chem 1996; 271: 27109–27112.
Andrade F, Roy S, Nicholson D, Thornberry N, Rosen A, Casciola-Rosen L . Granzyme B directly and efficiently cleaves several downstream caspase substrates: implications for CTL-induced apoptosis. Immunity 1998; 8: 451–460.
Heibein JA, Goping IS, Barry M, Pinkoski MJ, Shore GC, Green DR et al. Granzyme B-mediated cytochrome c release is regulated by the Bcl-2 family members Bid en Bax. J Exp Med 2000; 192: 1391–1401.
Monaco JJ . A molecular model MHC class-I-restricted antigen presentation. Immunol Today 1992; 13: 173–179.
Rees C, Mian S . Selective MHC expression in tumors modulates adaptive and innate antitumor responses. Cancer Immunol Immunother 1999; 48: 374–381.
Riemersma SA, Jordanova ES, Schop RFJ, Philippo K, Looijenga LHJ, Schuuring E et al. Extensive genetic alterations of the HLA region, including homozygous deletions of HLA class II genes in B-cell lymphomas arising in immune-privileged sites. Blood 2000; 96: 3569–3577.
Frisan T, Zhang QJ, Levitskaya J, Coram M, Kurilla MG, Masucci MG . Defective presentation of MHC class I-restricted cytotoxic T-cell epitopes in Burkitt's lymphoma cells. Int J Cancer 1996; 68: 251–258.
Oudejans JJ, Jiwa NM, Kummer JA, Horstman A, Vos W, Baak JP et al. Analysis of major histocompatibility complex class I expression in Reed–Sternberg cells in relation to the cytotoxic T-cell response in Epstein–Barr virus positive and negative Hodgkin's disease. Blood 1996; 87: 38–44.
Murray PG, Constandinou CM, Crocker J, Young LS, Ambinder RF . Analysis of major histocompatibility complex class I, TAP expression, and LMP2 epitope sequence in Epstein–Barr virus-positive Hodgkin's disease. Blood 1998; 92: 2477–2483.
Bird CH, Sutton VR, Sun J, Hirst CE, Novak A, Kumar S et al. Selective regulation of apoptosis: the cytotoxic lymphocyte serpin proteinase inhibitor 9 protects against granzyme B-mediated apoptosis without perturbing the Fas cell death pathway. Mol Cell Biol 1998; 18: 6387–6398.
Sun J, Bird CH, Sutton V, Coughlin PB, De Jong TA, Trapani JA et al. A cytosolic granzyme B inhibitor related to the viral apoptotic regulator cytokine response modifier A is present in cytotoxic lymphocytes. J Biol Chem 1996; 271: 27802–27809.
Bladergroen BA, Meijer CJLM, Ten Berge RL, Hack CE, Muris JJF, Dukers DF et al. Expression of the granzyme B inhibitor, protease inhibitor 9, by tumor cells in patients with Hodgkin's and non-Hodgkin's lymphoma: a novel protective mechanism for tumor cells to circumvent the immune system? Blood 2002; 99: 232–237.
Gospodarowicz MK, Sutcliffe SB . The extranodal lymphomas. Semin Radiat Oncol 1995; 4: 281–300.
Kummer JA, Kamp AM, van Katwijk M, Brakenhoff JP, Radosevic K, van Leeuwen AM et al. Production and characterization of monoclonal antibodies raised against recombinant human granzymes A and B and showing cross reactions with the natural proteins. J Immunol Methods 1993; 163: 77–83.
Stam NJ, Vroom TM, Peters PJ, Pastoors EB, Ploegh HL . HLA-A and HLA-B-specific monoclonal antibodies reactive with free heavy chains in Western blots, in formalin fixed, paraffin embedded tissue sections and in cryo-immuno-electron microscopy. Int Immunol 1990; 2: 113–125.
Polkowski W, Meijer GA, Baak JP, ten Kate FJ, Obertop H, Offerhaus GJ et al. Reproducibility of p53 and Ki-67 immunoquantitation in Barrett's esophagus. Anal Quant Cytol Histol 1997; 19: 246–254.
Cox DR . Regression models and life tables. J R Stat Soc Br 34; 187: 1972.
Armitage JO . Weisenberger DD for the non-Hodgkin's lymphoma classification project. New approach to classifying non-Hodgkin's lymphomas. Clinical features of the major histologic subtypes. J Clin Oncol 1998; 16: 2780–2795.
Schmitt CA, Lowe SW . Apoptosis and therapy. J Pathol 1999, 127–137.
Friesen C, Herr I, Krammer PH, Debatin KM . Involvement of the CD95 (APO-1/FAS) receptor/ligand system in drug-induced apoptosis in leukemia cells. Nat Med 1996; 2: 574–577.
Los M, Herr I, Friesen C, Fulda S, Schulze-Osthoff K, Debatin KM . Cross-resistance of CD95- and drug-induced apoptosis as a consequence of deficient activation of caspases 9 ICE/Ced-3 proteases). Blood 1997; 90: 3118–3129.
Friesen C, Fulda S, Debatin KM . Induction of CD95 ligand and apoptosis by doxirubicin is modulated by the redox state in chemosensitive- and drug-resistant tumor cells. Cell Death Differ 1999; 6: 471–480.
Boesen-de Cock JG, Tepper AD, de Vries E, van Blitterswijk WJ, Borst J . Common regulation of apoptosis signaling induced by CD95 and the DNA-damaging stimuli etoposide and gamma-radiation downstream from caspase-8 activation. J Biol Chem 1999; 274: 14255–14261.
Felgar RE, Greer JP, Macon W . T-cell-rich large-B-cell lymphoma (TCRBCL) as compared to diffuse large-B-cell lymphoma [letter]. Am J Pathol 1998; 153: 1707–1715.
Wolkers MC, Stoetter G, Vyth-Dreese FA, Schumacher TNM . Redundancy of direct priming and cross-priming in tumor specific CD8+ T cell responses. J Immunol 2001; 67: 3577–3584.
Heath WR, Carbone FR . Cross-presentation, dendritic cells, tolerance and immunity [review]. Annu Rev Immunol 2001; 19: 47–64.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Muris, J., Meijer, C., Cillessen, S. et al. Prognostic significance of activated cytotoxic T-lymphocytes in primary nodal diffuse large B-cell lymphomas. Leukemia 18, 589–596 (2004). https://doi.org/10.1038/sj.leu.2403240
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.leu.2403240
Keywords
This article is cited by
-
A novel angiogenesis-related scoring model predicts prognosis risk and treatment responsiveness in diffuse large B-cell lymphoma
Clinical and Experimental Medicine (2023)
-
Immune infiltration could predict the efficacy of short-term radiotherapy in patients with cervical cancer
Clinical and Translational Oncology (2022)
-
Dynamic changes in peripheral blood lymphocyte subset counts and functions in patients with diffuse large B cell lymphoma during chemotherapy
Cancer Cell International (2021)
-
The clinical role of the TME in solid cancer
British Journal of Cancer (2019)
-
Integrating histopathology, immune biomarkers, and molecular subgroups in solid cancer: the next step in precision oncology
Virchows Archiv (2019)