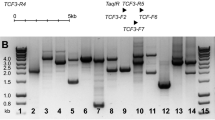
Abstract
The t(12;21) translocation resulting in the TEL–AML1 gene fusion is found in 25% of childhood B-cell precursor (BCP) acute lymphoblastic leukemias (ALL). Since TEL–AML1 has been reported to induce cell cycle retardation and thus may influence somatic recombination, we analyzed 214 TEL–AML1-positive ALL by PCR for rearrangements of the immunoglobulin (Ig) and T-cell receptor (TCR) genes. As a control group, 174 childhood BCP ALL without a TEL–AML1 were used. The majority of TEL–AML1-positive leukemias had a higher number of Ig/TCR rearrangements than control ALL. They also had a more mature immunogenotype characterized by their high frequency of complete IGH, IGK-Kde, and TCRG rearrangements. While IGK-Kde and TCRG were more frequently rearranged on both alleles at higher age, IGH and TCRD rearrangements decreased in their incidence along with a decrease in biallelic IGH rearrangements. This suggests that the recombination process continues in these leukemias leading to ongoing rearrangements and possibly also deletions of antigen receptor genes. We here provide first evidence that somatic recombination of antigen receptor genes is affected by the TEL–AML1 fusion, and that further age-related differences are probably caused by the longer latency period of the prenatally initiated TEL–AML1-positive leukemias in older children.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Romana SP, Poirel H, Leconiat M, Flexor MA, Mauchauffe M, Jonveaux P . High frequency of t(12;21) in childhood B-lineage acute lymphoblastic leukemia. Blood 1995; 86: 4263–4269.
Golub TR, Barker GF, Bohlander SK, Hiebert SW, Ward DC, Bray-Ward P . Fusion of the TEL gene on 12p13 to the AML1 gene on 21q22 in acute lymphoblastic leukemia. Proc Natl Acad Sci USA 1995; 92: 4917–4921.
Romana SP, Mauchauffe M, Le Coniat M, Chumakov I, Le Paslier D, Berger R . The t(12;21) of acute lymphoblastic leukemia results in a TEL–AML1 gene fusion. Blood 1995; 85: 3662–3670.
Shurtleff SA, Buijs A, Behm FG, Rubnitz JE, Raimondi SC, Hancock ML . TEL/AML1 fusion resulting from a cryptic t(12;21) is the most common genetic lesion in pediatric ALL and defines a subgroup of patients with an excellent prognosis. Leukemia 1995; 9: 1985–1989.
Borkhardt A, Cazzaniga G, Viehmann S, Valsecchi MG, Ludwig WD, Burci L . Incidence and clinical relevance of TEL/AML1 fusion genes in children with acute lymphoblastic leukemia enrolled in the German and Italian multicenter therapy trials Associazione Italiana Ematologia Oncologia Pediatrica and the Berlin–Frankfurt–Munster Study Group. Blood 1997; 90: 571–577.
Greaves M . Molecular genetics, natural history and the demise of childhood leukaemia. Eur J Cancer 1999; 35: 173–185.
Ford AM, Bennett CA, Price CM, Bruin MC, van Wering ER, Greaves M . Fetal origins of the TEL–AML1 fusion gene in identical twins with leukemia. Proc Natl Acad Sci USA 1998; 95: 4584–4588.
Wiemels JL, Ford AM, van Wering ER, Postma A, Greaves M . Protracted and variable latency of acute lymphoblastic leukemia after TEL–AML1 gene fusion in utero. Blood 1999; 94: 1057–1062.
Maia AT, Ford AM, Jalali GR, Harrison CJ, Taylor GM, Eden OB . Molecular tracking of leukemogenesis in a triplet pregnancy. Blood 2001; 98: 478–482.
Wiemels JL, Cazzaniga G, Daniotti M, Eden OB, Addison GM, Masera G . Prenatal origin of acute lymphoblastic leukaemia in children. Lancet 1999; 354: 1499–1503.
Lin WC, Desiderio S . V(D)J recombination and the cell cycle. Immunol Today 1995; 16: 279–289.
Lin WC, Desiderio S . Cell cycle regulation of V(D)J recombination-activating protein RAG-2. Proc Natl Acad Sci USA 1994; 91: 2733–2737.
Alt FW, Oltz EM, Young F, Gorman J, Taccioli G, Chen J . VDJ recombination. Immunol Today 1992; 13: 306–314.
van der Burg M, Barendregt BH, Szczepanski T, van Wering ER, Langerak AW, van Dongen JJ . Immunoglobulin light chain gene rearrangements display hierarchy in absence of selection for functionality in precursor-B-ALL. Leukemia 2002; 16: 1448–1453.
Aubin J, Davi F, Nguyen-Salomon F, Leboeuf D, Debert C, Taher M . Description of a novel FR1 IgH PCR strategy and its comparison with three other strategies for the detection of clonality in B cell malignancies. Leukemia 1995; 9: 471–479.
Szczepanski T, Willemse MJ, van Wering ER, van Weerden JF, Kamps WA, van Dongen JJ . Precursor-B-ALL with D(H)-J(H) gene rearrangements have an immature immunogenotype with a high frequency of oligoclonality and hyperdiploidy of chromosome 14. Leukemia 2001; 15: 1415–1423.
Beishuizen A, Verhoeven MA, Mol EJ, van Dongen JJ . Detection of immunoglobulin kappa light-chain gene rearrangement patterns by Southern blot analysis. Leukemia 1994; 8: 2228–2236.
Szczepanski T, Beishuizen A, Pongers-Willemse MJ, Hahlen K, van Wering ER, Wijkhuijs AJ . Cross-lineage T cell receptor gene rearrangements occur in more than ninety percent of childhood precursor-B acute lymphoblastic leukemias: alternative PCR targets for detection of minimal residual disease. Leukemia 1999; 13: 196–205.
Wasserman R, Galili N, Ito Y, Reichard BA, Shane S, Rovera G . Predominance of fetal type DJH joining in young children with B precursor lymphoblastic leukemia as evidence for an in utero transforming event. J Exp Med 1992; 176: 1577–1581.
Steenbergen EJ, Verhagen OJ, van Leeuwen EF, Behrendt H, Merle PA, Wester MR . B precursor acute lymphoblastic leukemia third complementarity-determining regions predominantly represent an unbiased recombination repertoire: leukemic transformation frequently occurs in fetal life. Eur J Immunol 1994; 24: 900–908.
Schneider M, Panzer S, Stolz F, Fischer S, Gadner H, Panzer-Grumayer ER . Crosslineage TCR delta rearrangements occur shortly after the DJ joinings of the IgH genes in childhood precursor B ALL and display age-specific characteristics. Br J Haematol 1997; 99: 115–121.
Szczepanski T, Langerak AW, Wolvers-Tettero IL, Ossenkoppele GJ, Verhoef G, Stul M . Immunoglobulin and T cell receptor gene rearrangement patterns in acute lymphoblastic leukemia are less mature in adults than in children: implications for selection of PCR targets for detection of minimal residual disease. Leukemia 1998; 12: 1081–1088.
Wiemels JL, Leonard BC, Wang Y, Segal MR, Hunger SP, Smith MT . Site-specific translocation and evidence of postnatal origin of the t(1;19) E2A-PBX1 fusion in childhood acute lymphoblastic leukemia. Proc Natl Acad Sci USA 2002; 99: 15101–15106.
Brumpt C, Delabesse E, Beldjord K, Davi F, Cayuela JM, Millien C . The incidence of clonal T-cell receptor rearrangements in B-cell precursor acute lymphoblastic leukemia varies with age and genotype. Blood 2000; 96: 2254–2261.
Fasching K, Panzer S, Haas OA, Borkhardt A, Marschalek R, Griesinger F . Presence of N regions in the clonotypic DJ rearrangements of the immunoglobulin heavy-chain genes indicates an exquisitely short latency in t(4;11)-positive infant acute lymphoblastic leukemia. Blood 2001; 98: 2272–2274.
Peham M, Panzer S, Fasching K, Haas OA, Fischer S, Marschalek R . Low frequency of clonotypic Ig and T-cell receptor gene rearrangements in t(4;11) infant acute lymphoblastic leukaemia and its implication for the detection of minimal residual disease. Br J Haematol 2002; 117: 315–321.
Yagi T, Hibi S, Tabata Y, Kuriyama K, Teramura T, Hashida T . Detection of clonotypic IGH and TCR rearrangements in the neonatal blood spots of infants and children with B-cell precursor acute lymphoblastic leukemia. Blood 2000; 96: 264–268.
Bernardin F, Friedman AD . AML1 stimulates G1 to S progression via its transactivation domain. Oncogene 2002; 21: 3247–3252.
Hiebert SW, Sun W, Davis JN, Golub T, Shurtleff S, Buijs A . The t(12;21) translocation converts AML-1B from an activator to a repressor of transcription. Mol Cell Biol 1996; 16: 1349–1355.
Willems P, Verhagen O, Segeren C, Veenhuizen P, Guikema J, Wiemer E . Consensus strategy to quantitate malignant cells in myeloma patients is validated in a multicenter study. Belgium-Dutch Hematology–Oncology Group. Blood 2000; 96: 63–70.
Szczepanski T, Pongers-Willemse MJ, Langerak AW, Harts WA, Wijkhuijs AJ, van Wering ER . Ig heavy chain gene rearrangements in T-cell acute lymphoblastic leukemia exhibit predominant DH6-19 and DH7-27 gene usage, can result in complete V-D-J rearrangements, and are rare in T-cell receptor alpha beta lineage. Blood 1999; 93: 4079–4085.
Pongers-Willemse MJ, Seriu T, Stolz F, d'Aniello E, Gameiro P, Pisa P . Primers and protocols for standardized detection of minimal residual disease in acute lymphoblastic leukemia using immunoglobulin and T cell receptor gene rearrangements and TAL1 deletions as PCR targets: report of the BIOMED-1 CONCERTED ACTION: investigation of minimal residual disease in acute leukemia. Leukemia 1999; 13: 110–118.
Konrad M, Metzler M, Panzer S, Ostreicher I, Peham M, Repp R . Late relapses evolve from slow-responding subclones in t(12;21)-positive acute lymphoblastic leukemia: evidence for the persistence of a preleukemic clone. Blood 2003; 101: 3635–3640.
Raimondi SC, Pui CH, Hancock ML, Behm FG, Filatov L, Rivera GK . Heterogeneity of hyperdiploid (51-67) childhood acute lymphoblastic leukemia. Leukemia 1996; 10: 213–224.
Height SE, Swansbury GJ, Matutes E, Treleaven JG, Catovsky D, Dyer MJ . Analysis of clonal rearrangements of the Ig heavy chain locus in acute leukemia. Blood 1996; 87: 5242–5250.
Dyer MJ, Heward JM, Zani VJ, Buccheri V, Catovsky D . Unusual deletions within the immunoglobulin heavy-chain locus in acute leukemias. Blood 1993; 82: 865–871.
Hansen-Hagge TE, Yokota S, Reuter HJ, Schwarz K, Bartram CR . Human common acute lymphoblastic leukemia-derived cell lines are competent to recombine their T-cell receptor delta/alpha regions along a hierarchically ordered pathway. Blood 1992; 80: 2353–2362.
Greaves MF, Chan LC, Furley AJ, Watt SM, Molgaard HV . Lineage promiscuity in hemopoietic differentiation and leukemia. Blood 1986; 67: 1–11.
Beishuizen A, Hahlen K, Hagemeijer A, Verhoeven MA, Hooijkaas H, Adriaansen HJ . Multiple rearranged immunoglobulin genes in childhood acute lymphoblastic leukemia of precursor B-cell origin. Leukemia 1991; 5: 657–667.
Jabber Al-Obaidi MS, Martineau M, Bennett CF, Franklin IM, Goldstone AH, Harewood L . ETV6/AML1 fusion by FISH in adult acute lymphoblastic leukemia. Leukemia 2002; 16: 669–674.
Aguiar RC, Sohal J, van Rhee F, Carapeti M, Franklin IM, Goldstone AH . TEL–AML1 fusion in acute lymphoblastic leukaemia of adults. M.R.C. Adult Leukaemia Working Party. Br J Haematol 1996; 95: 673–677.
Van Zant G, de Haan G, Rich IN . Alternatives to stem cell renewal from a developmental viewpoint. Exp Hematol 1997; 25: 187–192.
Acknowledgements
We thank Uli Monschein, Lilia Corral, Elisabetta d'Aniello, Simona Songia, Monica Spinelli, Giuseppe Germano, Laura DelGiudice, Annemarie Wijkhuijs, Simone Busenbender, Ivonne Gonnissen, Carmen Holzschuh, Heike Knappenschneider, Kirsten Linsmeier, Nicola Passow, and Cornelia Rütz for their excellent technical assistance, and Christos Diakos for fruitful discussions. The study was performed using the network of the Biology group within the I-BFM group. This study was supported by a grant from the Fonds zur Förderung Wissenschaftlicher Forschung (FWF P-13575-MED), by the Österreichische Kinderkrebshilfe, by the Fondazione MTettamanti, Fondazione Cariplo, Fondazione “Città della speranza”, Associazione Italiana per la Ricerca sul Cancro (AIRC), CNR-MIUR Progetto Finalizzato Oncologia, by a grant from the Deutsche Krebshilfe, and by the Dutch Cancer Society/Koningin Wilhelmina Fonds (Grants SNWLK 97-1567 and SNWLK 2000-2268).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hübner, S., Cazzaniga, G., Flohr, T. et al. High incidence and unique features of antigen receptor gene rearrangements in TEL–AML1-positive leukemias. Leukemia 18, 84–91 (2004). https://doi.org/10.1038/sj.leu.2403182
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.leu.2403182
Keywords
This article is cited by
-
In Utero Development and Immunosurveillance of B Cell Acute Lymphoblastic Leukemia
Current Treatment Options in Oncology (2022)
-
ETV6-RUNX1 and RUNX1 directly regulate RAG1 expression: one more step in the understanding of childhood B-cell acute lymphoblastic leukemia leukemogenesis
Leukemia (2022)
-
Prognostic value and clinical significance of TCR rearrangements for MRD monitoring in ALL patients
Comparative Clinical Pathology (2017)
-
Chromosome 14 copy number-dependent IGH gene rearrangement patterns in high hyperdiploid childhood B-cell precursor ALL: implications for leukemia biology and minimal residual disease analysis
Leukemia (2009)
-
Acute lymphoblastic leukemia with t(4;11) in children 1 year and older: The ‘big sister’ of the infant disease?
Leukemia (2007)