Abstract
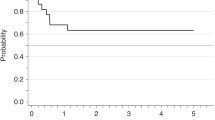
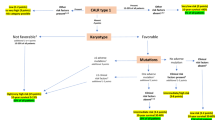
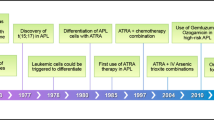
With improved treatment of acute promyelocytic leukemia (APL) by all trans retinoic acid (ATRA) combined to anthracycline–aracytin chemotherapy (CT), a larger number of those patients may be at risk of late complications. Recently, the Rome group reported five cases of myelodysplastic syndrome (MDS) or acute myeloid leukemia (AML, non-APL) occurring during the course of 77 APL patients (6.5%) in complete remission (CR). From 1991 to 1998, we treated 677 newly diagnosed cases of APL, and 617 of them achieved CR with ATRA combined to CT (n=579) or CT alone (n=38); 246 of them received subsequent maintenance CT with 6 mercaptopurine and methotrexate. With a median follow-up of 51 months, 6 patients (0.97%) developed MDS, 13–74 months after the diagnosis of APL. In all six cases, t(15;17) and PML-RARalpha rearrangement were absent at the time of MDS diagnosis, and karyotype mainly showed complex cytogenetic abnormalities involving chromosomes 5 and/or 7, typical of MDS observed after treatment with alkylating agents, although none of the six patients had received such agents for the treatment of APL. Our findings suggest that MDS can indeed be a long-term complication in APL, although probably at lower incidence than that previously reported.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Bennett JM, Catovsky D, Daniel MT, Flandrin G, Galton DA, Gralnick HR et al. Proposals for the classification of the acute leukaemias. French–American–British (FAB) co-operative group. Br J Haematol 1976; 33: 451–458.
Bennett JM, Catovsky D, Daniel MT, Flandrin G, Galton DA, Gralnick HR et al. A variant form of hypergranular promyelocytic leukemia (M3). Ann Intern Med 1980; 92: 261.
De Botton S, Chevret S, Sanz M, Dombret H, Thomas X, Guerci A . Additional chromosomal abnormalities in patients with acute promyelocytic leukaemia (APL) do not confer poor prognosis: results of APL 93 trial. Br J Haematol. 2000; 111: 801–806.
Fenaux P, Chomienne C, Degos L . All-trans retinoic acid and chemotherapy in the treatment of acute promyelocytic leukemia. Semin Hematol 2001; 38: 13–25.
Sanz MA, Martin G, Rayon C, Esteve J, Gonzalez M, Diaz-Mediavilla J et al. A modified AIDA protocol with anthracycline-based consolidation results in high antileukemic efficacy and reduced toxicity in newly diagnosed PML/RARalpha-positive acute promyelocytic leukemia. PETHEMA group. Blood 1999; 94: 3015–3021.
Tallman MS, Andersen JW, Schiffer CA, Appelbaum FR, Feusner JH, Woods WG et al. All-trans retinoic acid in acute promyelocytic leukemia: long-term outcome and prognostic factor analysis from the North American Intergroup protocol. Blood 2002; 100: 4298–4302.
Latagliata R, Petti MC, Fenu S, Mancini M, Spiriti MA, Breccia M et al. Therapy-related myelodysplastic syndrome–acute myelogenous leukemia in patients treated for acute promyelocytic leukaemia: an emerging problem. Blood 2002; 99: 822–824.
Stasi R, Taylor CG, Venditti A, Del Poeta G, Aronica G, Bastianelli C et al. Contribution of immunophenotypic and genotypic analyses to the diagnosis of acute leukemia. Ann Hematol 1995; 71: 13–27.
Fenaux P, Le Deley MC, Castaigne S, Archimbaud E, Chomienne C, Link H et al. Effect of all transretinoic acid in newly diagnosed acute promyelocytic leukemia. Results of a multicenter randomized trial. European APL 91 Group. Blood 1993; 82: 3241–3249.
Fenaux P, Chastang C, Chomienne C, Castaigne S, Sanz M, Link H et al. Treatment of newly diagnosed acute promyelocytic leukemia (APL) by all transretinoic acid (ATRA) combined with chemotherapy: the European experience. European APL Group. Leuk Lymphoma 1995; 16: 431–437.
Fenaux P, Chastang C, Chevret S, Sanz M, Dombret H, Archimbaud E et al. A randomized comparison of all transretinoic acid (ATRA) followed by chemotherapy and ATRA plus chemotherapy and the role of maintenance therapy in newly diagnosed acute promyelocytic leukemia. The European APL Group. Blood 1999; 94: 1192–1200.
Bennett JM, Catovsky D, Daniel MT, Flandrin G, Galton DA, Gralnick HR et al. Proposals for the classification of the myelodysplastic syndromes. Br J Haematol 1982; 51: 189–199.
Zompi S, Legrand O, Bouscary D, Blanc CM, Picard F, Casadevall N et al. Therapy-related acute myeloid leukaemia after successful therapy for acute promyelocytic leukaemia with t(15;17): a report of two cases and a review of the literature. Br J Haematol 2000; 110: 610–613.
Zompi S, Viguie F . Therapy-related acute myeloid leukemia and myelodysplasia after successful treatment of acute promyelocytic leukemia. Leuk Lymphoma 2002; 43: 275–280.
Jubashi T, Nagai K, Miyazaki Y, Nakamura H, Matsuo T, Kuriyama K et al. A unique case of t(15;17) acute promyelocytic leukaemia (M3) developing into acute myeloblastic leukaemia (M1) with t(7;21) at relapse. Br J Haematol 1993; 83: 665–668.
Miyazaki H, Ino T, Sobue R, Kojima H, Wakita M, Nomura T et al. Translocation (3;21)(q26;q22) in treatment-related acute leukemia secondary to acute promyelocytic leukemia. Cancer Genet Cytogenet 1994; 74: 84–86.
Hatzis T, Standen GR, Howell RT, Savill C, Wagstaff M, Scott GL . Acute promyelocytic leukaemia (M3): relapse with acute myeloblastic leukaemia (M2) and dic(5;17) (q11;p11). Am J Hematol 1995; 48: 40–44.
Todisco E, Testi AM, Avvisati G, Moleti ML, Cedrone M, Cimino G et al. Therapy-related acute myelomonocytic leukemia following successful treatment for acute promyelocytic leukemia. Leukemia 1995; 9: 1583–1585.
Bseiso AW, Kantarjian H, Estey E . Myelodysplastic syndrome following successful therapy of acute promyelocytic leukemia. Leukemia 1997; 11: 168–169.
Meloni G, Diverio D, Vignetti M, Avvisati G, Capria S, Petti MC et al. Autologous bone marrow transplantation for acute promyelocytic leukemia in second remission: prognostic relevance of pretransplant minimal residual disease assessment by reverse-transcription polymerase chain reaction of the PML/RAR alpha fusion gene. Blood 1997; 90: 1321–1325.
Sawada H, Morimoto H, Wake A, Yamasaki Y, Izumi Y . Therapy-related acute myeloid leukemia with t(10;11)(q23;p15) following successful chemotherapy for acute promyelocytic leukemia with t(15;17)(q22;q21). Int J Hematol 1999; 69: 270–271.
Stavroyianni N, Yataganas X, Abazis D, Pangalos C, Meletis J . Acute promyelocytic leukemia relapsing into FAB-M2 acute myeloid leukemia with trisomy 8. Cancer Genet Cytogenet 2000; 117: 82–83.
Au WY, Lam CC, Ma ES, Man C, Wan T, Kwong YL . Therapy-related myelodysplastic syndrome after eradication of acute promyelocytic leukemia: cytogenetic and molecular features. Hum Pathol 2001; 32: 126–129.
Felice MS, Rossi J, Gallego M, Zubizarreta PA, Cygler AM, Alfaro E et al. Acute trilineage leukemia with monosomy of chromosome 7 following an acute promyelocytic leukemia. Leuk Lymphoma 1999; 34: 409–413.
Pecci A, Invernizzi R . A therapy-related myelodysplastic syndrome with unusual features in a patient treated for acute promyelocytic leukemia. Haematologica 2001; 86: 102–103.
Pedersen-Bjergaard J, Philip P, Larsen SO, Jensen G, Byrsting K . Chromosome aberrations and prognostic factors in therapy-related myelodysplasia and acute nonlymphocytic leukaemia. Blood 1990; 76: 1083–1091.
Pedersen-Bjergaard J, Pedersen M, Roulston D, Philip P . Different genetic pathways in leukemogenesis for patients presenting with therapy-related myelodysplasia and therapy-related acute myeloid leukemia. Blood 1995; 86: 3542–3552.
Lo Coco F, Latagliata R, Diverio D, Breccia M, Chiusolo P, Mandelli F . Independent clonal origin of therapy-related MDS-AML developing after treatment of acute promyelocytic leukemia. Blood 2002; 100: 1929.
Klarskov Andersen M, Pedersen-Bjergaard J . Therapy-related MDS and AML in acute promyelocytic leukemia. Blood 2002; 100: 1928–1929.
Acknowledgements
This work was supported by the Ligue Nationale Centre le Cancer (Comilé du Nord). The Association de Accheriche Centre le Cancer and the Programme Hospitalion de Recherche Clinique.
Author information
Authors and Affiliations
Consortia
Corresponding author
Rights and permissions
About this article
Cite this article
Lobe, I., Rigal-Huguet, F., Vekhoff, A. et al. Myelodysplastic syndrome after acute promyelocytic leukemia: the European APL group experience. Leukemia 17, 1600–1604 (2003). https://doi.org/10.1038/sj.leu.2403034
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.leu.2403034
Keywords
This article is cited by
-
Incidence, risk factors, and outcomes of second neoplasms in patients with acute promyelocytic leukemia: the PETHEMA-PALG experience
Annals of Hematology (2024)
-
Evolution of NPM1-negative therapy-related myelodysplastic syndromes following curative treatment of NPM1-mutant AML
Leukemia (2017)
-
Chronic Myeloid Leukemia Developing After Successful Treatment of Acute Promyelocytic Leukemia
Indian Journal of Hematology and Blood Transfusion (2016)
-
Unraveling Myelodysplastic Syndromes: Current Knowledge and Future Directions
Current Oncology Reports (2016)
-
Loss of response to azacitidine is associated with deletion 12p13 in a patient with myelodysplastic syndrome with unique translocation t(13;17)(q12;q25) after prior breast cancer and acute promyelocytic leukemia
Annals of Hematology (2015)