Abstract
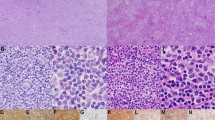
The translocation (14;18)(q32;q21) is the hallmark of follicular lymphoma (FL). However, conventional cytogenetics and PCR techniques fail to detect it in at least 10% of cases. In order to evaluate the true incidence of this translocation in FL, we analyzed 63 patients with FL, and 17 patients with diffuse large cell lymphoma (DLCL) corresponding to suspected FL transformations using interphase fluorescence in situ hybridization (FISH). Colocalized signals related to the translocation were observed in 19–92% of cells (median = 51%), corresponding to positivity over the threshold in all (63/63) cases. Similarly, 16/17 possibly secondary DLCL displayed the translocation. Although some cytogenetic changes might be missed by this FISH assay (such as rare insertion, or translocations with other chromosomal partners), our results stress t(14;18)(q32;q21) as an almost constant finding in FL. Our sensitive interphase FISH assay should be of great value to define FL more accurately, namely in patients included into therapeutic trials. Furthermore, this approach could be of interest in (re)defining some types of FL, especially the grade 3 FL which frequently lack t(14;18).
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
The Non-Hodgkin's Lymphoma Classification Project A clinical trial of the International Lymphoma Study Group classification of non-Hodgkin's lymphoma. Blood, (1997). 89, 3909–3918.
Harris, NL, Jaffe, ES, Diebold, J, Flandrin, G, Muller-Hermelink, HK, Vardiman, J, Lister, TA & Bloomfield, CD The World Health Organization Classification of Neoplasms of the Hematopoietic and Lymphoid Tissues: Report of the Clinical Advisory Commitee Meeting – Airlie House, Virginia, November, 1997. The Hematology Journal, (2000). 1, 53–56.
Macintyre, E, Willerford, D & Morris, SW Non-Hodgkin's Lymphoma: molecular features of B Cell Lymphoma. Education Program Book of the American Society of Hematology, American Society of Hematology: San Francisco (2000). pp180–204.
Skinnider, BF, Horsman, DE, Dupuis, B & Gascoyne, RD Bcl-6 and Bcl-2 protein expression in diffuse large B-cell lymphoma and follicular lymphoma: correlation with 3q27 and 18q21 chromosomal abnormalities. Hum Pathol, (1999). 30, 803–808.
Lopez-Guillermo, A, Cabanillas, F, McDonnell, TI, McLaughlin, P, Smith, T, Pugh, W, Hagemeister, F, Rodriguez, MA, Romaguera, JE, Younes, A, Sarris, AH, Preti, HA & Lee, MS Correlation of bcl-2 rearrangement with clinical characteristics and outcome in indolent follicular lymphoma. Blood, (1999). 93, 3081–3087.
Vaandrager, JW, Schuuring, E, Raap, T, Philippo, K, Kleiverda, K & Kluin, P Interphase FISH detection of BCL2 rearrangement in follicular lymphoma using breakpoint-flanking probes. Genes Chromosomes Cancer, (2000). 27, 85–94.
Poetsch, M, Weber-Matthiesen, K, Plendl, HJ, Grote, W & Schlegelberger, B Detection of the t(14;18) chromosomal translocation by interphase cytogenetics with yeast-artificial-chromosome probes in follicular lymphoma and nonneoplastic lymphoproliferation. J Clin Oncol, (1996). 14, 963–969.
Akiko, T, Ikuo, M, Shinsuke, I, Shohei, Y, Shigeo, H, Kazuhiro, N, Hiroshi, F, Shigeo, N, Masao, S, Ryuzo, U & Masafumi, T Interphase detection of immunoglobulin heavy chain gene translocations with specific oncogene loci in 173 patients with B-cell lymphoma. Cancer Genet Cytogenet, (2001). 129, 1–9.
Mitelman, F, Johansson, B & Mertens, S Mitelman database of catalogue of chromosome aberrations in cancer. (2000). http://cgap.nci.nih.gov/Chromosomes/Mitelman
Knutsen, T Cytogenetic mechanisms in the pathogenesis and progression of follicular lymphoma. Cancer Surv, (1997). 30, 163–192.
Avet-Loiseau, H, Li, JY, Facon, T, Brigaudeau, C, Morineau, N, Maloisel, F, Rapp, MJ, Talmant, P, Trimoreau, F, Jaccard, A, Harousseau, JL & Bataille, R High incidence of translocations t(11;14)(q13;q32) and t(4;14)(p16;q32) in patients with plasma cells malignancies. Cancer Res, (1998). 58, 5640–5645.
Rimokh, R, Gadoux, M, Bertheas, MF, Berger, F, Garoscio, M, Deleage, G, Germain, D & Magaud, JP FVT-1, a novel human transcription unit affected by variant translocation t(2;18)(p11;q21) of follicular lymphoma. Blood, (1993). 81, 136–142.
Bastard, C, Tilly, H, Lenormand, B, Bigorgne, C, Boulet, D, Kunlin, A, Monconduit, M & Piguet, H Translocations involving band 3q27 and Ig gene regions in non-Hodgkin's lymphoma. Blood, (1992). 79, 2527–2531.
Gribben, JG, Saporito, L, Barber, M, Blake, KW, Edwards, RM, Griffin, JD, Freedman, AS & Nadler, LM Bone marrows of Non-Hodgkin's lymphoma patients with a bcl-2 translocation can be purged of polymerase chain reaction-detectable lymphoma cells using monoclonal antibodies and immunomagnetic bead depletion. Blood, (1992). 80, 1083–1089.
Vaandrager, JW, Schuuring, E, Philippo, K & Kluin, PM V(D)J recombinase-mediated transposition of the BCL2 gene to the IgH locus in follicular lymphoma. Blood, (2000). 96, 1647–1652.
Küppers, R, Klein, U, Hansmann, ML & Rajewsky, K Cellular origin of human B-cell lymphomas. N Engl J Med, (1999). 11, 1520–1529.
Buchonnet, G, Lenain, P, Ruminy, P, Lepretre, S, Stamatoullas, A, Parmentier, F, Jardin, F, Duval, C, Tilly, H & Bastard, C Characterization of BCL2-JH rearrangements in follicular lymphoma: PCR detection of 3’BCL2 breakpoints and evidence of a new cluster. Leukemia, (2000). 14, 1563–1569.
Yabumoto, K, Akasaka, T, Muramatsu, M, Kadowaki, M, Hayashi, T, Ohno, H, Fukuhara, S & Okuma, M Rearrangement of the 5’ cluster region of the BCL2 gene in lymphoid neoplasm: a summary of nine cases. Leukemia, (1996). 10, 970–977.
Seite, P, Hillion, J, d'Agay, MF, Berger, R & Larsen, CJ BCL2 complex rearrangement in follicular lymphoma: translocation mbr/JH and deletion in the vcr region of the same BCL2 allele. Oncogene, (1993). 8, 3073–3080.
Yunis, JJ, Frizzera, G, Oken, MM, McKenna, J, Theologides, A & Arnesen, M Multiple recurrent genomic defects in follicular lymphoma. A possible model for cancer. N Engl J Med, (1987). 316, 79–84.
Offit, K, Jhanwar, SC, Ladanyi, M, Filippa, DA & Chaganti, RS Cytogenetic analysis of 434 consecutively ascertained specimens of non-Hodgkin's lymphoma: correlations between recurrent aberrations, histology, and exposure to cytotoxic treatment. Genes Chromosomes Cancer, (1991). 3, 189–201.
Ott, G, Katzenberger, T, Lohr, A, Kindelberger, S, Rudiger, T, Wilhelm, M, Kalla, J, Rosenwald, A, Muller, JG, Ott, MM & Muller-Hermelink, HK Cytomorphologic, immunohistochemical, and cytogenetic profiles of follicular lymphoma: 2 types of follicular lymphoma grade 3. Blood, (2002). 99, 3806–3812.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Godon, A., Moreau, A., Talmant, P. et al. Is t(14;18)(q32;q21) a constant finding in follicular lymphoma? An interphase FISH study on 63 patients. Leukemia 17, 255–259 (2003). https://doi.org/10.1038/sj.leu.2402739
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.leu.2402739
Keywords
This article is cited by
-
Emerging molecular subtypes and therapeutic targets in B-cell precursor acute lymphoblastic leukemia
Frontiers of Medicine (2021)
-
A case–control study of tobacco use and other non-occupational risk factors for lymphoma subtypes defined by t(14; 18) translocations and bcl-2 expression
Cancer Causes & Control (2010)
-
The specificity of interphase FISH translocation probes in formalin fixed paraffin embedded tissue sections is readily assessed using automated staining and scoring of tissue microarrays constructed from murine xenografts
Journal of Molecular Histology (2007)